当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Isolation of Chemically Cyclized Peptide Binders Using Yeast Surface Display.
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2020-08-03 , DOI: 10.1021/acscombsci.0c00076 Kaitlyn Bacon 1 , Abigail Blain 1 , Matthew Burroughs 1 , Nikki McArthur 1 , Balaji M Rao 1, 2 , Stefano Menegatti 1, 2
ACS Combinatorial Science ( IF 3.903 ) Pub Date : 2020-08-03 , DOI: 10.1021/acscombsci.0c00076 Kaitlyn Bacon 1 , Abigail Blain 1 , Matthew Burroughs 1 , Nikki McArthur 1 , Balaji M Rao 1, 2 , Stefano Menegatti 1, 2
Affiliation
|
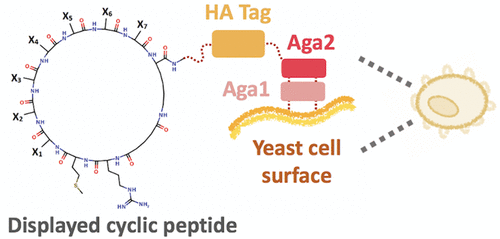
Cyclic peptides with engineered protein-binding activity have gained increasing attention for use in therapeutic and biotechnology applications. We describe the efficient isolation and characterization of cyclic peptide binders from genetically encoded combinatorial libraries using yeast surface display. Here, peptide cyclization is achieved by disuccinimidyl glutarate-mediated cross-linking of amine groups within a linear peptide sequence that is expressed as a yeast cell surface fusion. Using this approach, we first screened a library of cyclic heptapeptides using magnetic selection, followed by fluorescence activated cell sorting (FACS) to isolate binders for a model target (lysozyme) with low micromolar binding affinity (KD ∼ 1.2–3.7 μM). The isolated peptides bind lysozyme selectively and only when cyclized. Importantly, we showed that yeast surface displayed cyclic peptides can be used to efficiently obtain quantitative estimates of binding affinity, circumventing the need for chemical synthesis of the selected peptides. Subsequently, to demonstrate broader applicability of our approach, we isolated cyclic heptapeptides that bind human interleukin-17 (IL-17) using yeast-displayed IL-17 as a target for magnetic selection, followed by FACS using recombinant IL-17. Molecular docking simulations and follow-up experimental analyses identified a candidate cyclic peptide that likely binds IL-17 in its receptor binding region with moderate apparent affinity (KD ∼ 300 nM). Taken together, our results show that yeast surface display can be used to efficiently isolate and characterize cyclic peptides generated by chemical modification from combinatorial libraries.
中文翻译:

使用酵母表面展示分离化学环化肽结合剂。
具有工程化蛋白质结合活性的环肽在治疗和生物技术应用中的使用越来越受到关注。我们描述了使用酵母表面展示从遗传编码的组合文库中有效分离和表征环肽结合物。在这里,肽环化是通过二琥珀酰亚胺基戊二酸酯介导的线性肽序列内胺基的交联来实现的,该线性肽序列表达为酵母细胞表面融合物。使用这种方法,我们首先使用磁选筛选环状七肽文库,然后通过荧光激活细胞分选(FACS)来分离具有低微摩尔结合亲和力( K D ∼ 1.2–3.7 μM)的模型靶标(溶菌酶)的结合物。分离的肽仅在环化时选择性地结合溶菌酶。重要的是,我们表明酵母表面展示的环肽可用于有效地获得结合亲和力的定量估计,从而避免了对所选肽进行化学合成的需要。随后,为了证明我们的方法更广泛的适用性,我们使用酵母展示的 IL-17 作为磁力选择的靶标,分离了结合人白细胞介素 17 (IL-17) 的环七肽,然后使用重组 IL-17 进行 FACS。分子对接模拟和后续实验分析确定了一种候选环肽,它可能以中等表观亲和力(K D ∼ 300 nM)在其受体结合区域结合 IL-17。综上所述,我们的结果表明酵母表面展示可用于有效地分离和表征组合文库中通过化学修饰产生的环肽。
更新日期:2020-10-12
中文翻译:

使用酵母表面展示分离化学环化肽结合剂。
具有工程化蛋白质结合活性的环肽在治疗和生物技术应用中的使用越来越受到关注。我们描述了使用酵母表面展示从遗传编码的组合文库中有效分离和表征环肽结合物。在这里,肽环化是通过二琥珀酰亚胺基戊二酸酯介导的线性肽序列内胺基的交联来实现的,该线性肽序列表达为酵母细胞表面融合物。使用这种方法,我们首先使用磁选筛选环状七肽文库,然后通过荧光激活细胞分选(FACS)来分离具有低微摩尔结合亲和力( K D ∼ 1.2–3.7 μM)的模型靶标(溶菌酶)的结合物。分离的肽仅在环化时选择性地结合溶菌酶。重要的是,我们表明酵母表面展示的环肽可用于有效地获得结合亲和力的定量估计,从而避免了对所选肽进行化学合成的需要。随后,为了证明我们的方法更广泛的适用性,我们使用酵母展示的 IL-17 作为磁力选择的靶标,分离了结合人白细胞介素 17 (IL-17) 的环七肽,然后使用重组 IL-17 进行 FACS。分子对接模拟和后续实验分析确定了一种候选环肽,它可能以中等表观亲和力(K D ∼ 300 nM)在其受体结合区域结合 IL-17。综上所述,我们的结果表明酵母表面展示可用于有效地分离和表征组合文库中通过化学修饰产生的环肽。



























 京公网安备 11010802027423号
京公网安备 11010802027423号