当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical shifts of artificial monomers used to construct heterogeneous-backbone protein mimetics in random coil and folded states
Peptide Science ( IF 2.4 ) Pub Date : 2022-11-12 , DOI: 10.1002/pep2.24297 Shilpa R Rao 1 , Thomas W Harmon 1 , Shelby L Heath 1 , Jacob A Wolfe 1 , Jacqueline R Santhouse 1 , Gregory L O'Brien 1 , Alexis N Distefano 1 , Zachary E Reinert 1 , W Seth Horne 1
Peptide Science ( IF 2.4 ) Pub Date : 2022-11-12 , DOI: 10.1002/pep2.24297 Shilpa R Rao 1 , Thomas W Harmon 1 , Shelby L Heath 1 , Jacob A Wolfe 1 , Jacqueline R Santhouse 1 , Gregory L O'Brien 1 , Alexis N Distefano 1 , Zachary E Reinert 1 , W Seth Horne 1
Affiliation
|
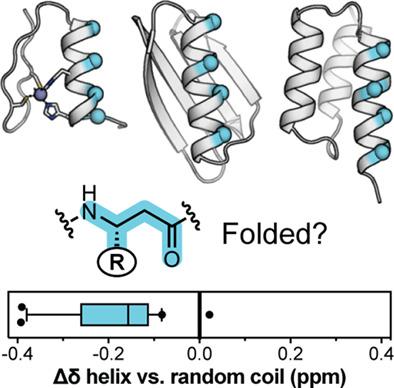
The construction of protein-sized synthetic chains that blend natural amino acids with artificial monomers to create so-called heterogeneous-backbones is a powerful approach to generate complex folds and functions from bio-inspired agents. A variety of techniques from structural biology commonly used to study natural proteins have been adapted to investigate folding in these entities. In NMR characterization of proteins, proton chemical shift is straightforward to acquire and an information-rich metric that bears directly on a variety of properties related to folding. Leveraging chemical shift to gain insight into folding requires a set of reference chemical shift values corresponding to each building block type (i.e., the 20 canonical amino acids in the case of natural proteins) in a random coil state and knowledge of systematic changes in chemical shift associated with particular folded conformations. Although well documented for natural proteins, these issues remain unexplored in the context of protein mimetics. Here, we report random coil chemical shift values for a library of artificial amino acid monomers frequently used to construct heterogeneous-backbone protein analogues as well as a spectroscopic signature associated with one monomer class, β3-residues bearing proteinogenic side chains, adopting a helical folded conformation. Collectively, these results will facilitate the continued utilization of NMR for the study of structure and dynamics in protein-like artificial backbones.
中文翻译:

用于构建随机卷曲和折叠状态异质骨架蛋白模拟物的人工单体的化学位移
蛋白质大小的合成链的构建将天然氨基酸与人工单体混合以创建所谓的异质主链,这是从仿生制剂中生成复杂折叠和功能的强大方法。结构生物学中常用来研究天然蛋白质的各种技术已被用来研究这些实体的折叠。在蛋白质的核磁共振表征中,质子化学位移很容易获取,并且是一种信息丰富的指标,直接影响与折叠相关的各种特性。利用化学位移来深入了解折叠需要一组与每个构建块类型相对应的参考化学位移值(即,天然蛋白质中的 20 种经典氨基酸)处于随机卷曲状态,并了解与特定折叠构象相关的化学位移的系统变化。尽管天然蛋白质有充分的记录,但这些问题在蛋白质模拟物的背景下仍未得到探索。在这里,我们报告了经常用于构建异质骨架蛋白类似物的人工氨基酸单体库的随机卷曲化学位移值,以及与一类单体相关的光谱特征,β 3 -带有蛋白质侧链的残基,采用螺旋折叠构象。总的来说,这些结果将有助于继续利用核磁共振来研究类蛋白质人工主链的结构和动力学。
更新日期:2022-11-12
中文翻译:

用于构建随机卷曲和折叠状态异质骨架蛋白模拟物的人工单体的化学位移
蛋白质大小的合成链的构建将天然氨基酸与人工单体混合以创建所谓的异质主链,这是从仿生制剂中生成复杂折叠和功能的强大方法。结构生物学中常用来研究天然蛋白质的各种技术已被用来研究这些实体的折叠。在蛋白质的核磁共振表征中,质子化学位移很容易获取,并且是一种信息丰富的指标,直接影响与折叠相关的各种特性。利用化学位移来深入了解折叠需要一组与每个构建块类型相对应的参考化学位移值(即,天然蛋白质中的 20 种经典氨基酸)处于随机卷曲状态,并了解与特定折叠构象相关的化学位移的系统变化。尽管天然蛋白质有充分的记录,但这些问题在蛋白质模拟物的背景下仍未得到探索。在这里,我们报告了经常用于构建异质骨架蛋白类似物的人工氨基酸单体库的随机卷曲化学位移值,以及与一类单体相关的光谱特征,β 3 -带有蛋白质侧链的残基,采用螺旋折叠构象。总的来说,这些结果将有助于继续利用核磁共振来研究类蛋白质人工主链的结构和动力学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号