NanoImpact ( IF 4.9 ) Pub Date : 2023-01-27 , DOI: 10.1016/j.impact.2023.100452 Daria Korejwo 1 , Savvina Chortarea 2 , Chrysovalanto Louka 2 , Marija Buljan 2 , Barbara Rothen-Rutishauser 3 , Peter Wick 2 , Tina Buerki-Thurnherr 2
|
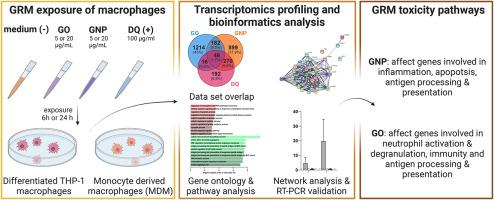
Graphene and its derivatives are attractive materials envisaged to enable a wealth of novel applications in many fields including energy, electronics, composite materials or health. A comprehensive understanding of the potential adverse effects of graphene-related materials (GRM) in humans is a prerequisite to the safe use of these promising materials. Here, we exploited gene expression profiling to identify transcriptional responses and toxicity pathways induced by graphene oxide (GO) and graphene nanoplatelets (GNP) in human macrophages. Primary human monocyte-derived macrophages (MDM) and a human macrophage cell line, i.e. differentiated THP-1 cells, were exposed to 5 or 20 μg/mL GO and GNP for 6 and 24 h to capture early and more persistent acute responses at realistic or slightly overdose concentrations. GO and GNP induced time-, dose- and macrophage type-specific differential expression of a substantial number of genes with some overlap between the two GRM types (up to 384 genes (9.6%) or 447 genes (20.4%) in THP-1 or MDM, respectively) but also a high number of genes exclusively deregulated from each material type. Furthermore, GRM responses on gene expression were highly different from those induced by inflammogenic material crystalline quartz (maximum of 64 (2.3%) or 318 (11.3%) common genes for MDM treated with 20 μg/mL GO and GNP, respectively). Further bioinformatics analysis revealed that GNP predominantly activated genes controlling inflammatory and apoptotic pathways whereas GO showed only limited inflammatory responses. Interestingly, both GRM affected the expression of genes related to antigen processing and presentation and in addition, GO activated pathways of neutrophil activation, degranulation and immunity in MDM. Overall, this study provides an extensive resource of potential toxicity mechanisms for future safety assessment of GRM in more advanced model systems to verify if the observed changes in gene expression in human macrophages could lead to long-term consequences on human health.
中文翻译:

氧化石墨烯和石墨烯纳米血小板处理后人类巨噬细胞的基因表达谱揭示了途径的颗粒特异性调节
石墨烯及其衍生物是极具吸引力的材料,有望在包括能源、电子、复合材料或健康在内的许多领域实现大量新颖的应用。全面了解石墨烯相关材料 (GRM) 对人类的潜在不利影响是安全使用这些有前途的材料的先决条件。在这里,我们利用基因表达谱来识别氧化石墨烯 (GO) 和石墨烯纳米血小板 (GNP) 在人类巨噬细胞中诱导的转录反应和毒性途径。原代人单核细胞来源的巨噬细胞 (MDM) 和人巨噬细胞系,即分化的 THP-1 细胞,暴露于 5 或 20 μg/mL GO 和 GNP 6 和 24 小时,以在现实情况下捕捉早期和更持久的急性反应或稍微过量的浓度。GO 和 GNP 诱导时间-, 大量基因的剂量和巨噬细胞类型特异性差异表达,两种 GRM 类型之间有一些重叠(THP-1 或 MDM 中分别高达 384 个基因(9.6%)或 447 个基因(20.4%)),但也大量基因专门从每种材料类型中解除管制。此外,GRM 对基因表达的反应与由致炎材料结晶石英诱导的反应高度不同(分别用 20 μg/mL GO 和 GNP 处理的 MDM 的最多 64 (2.3%) 或 318 (11.3%) 个常见基因)。进一步的生物信息学分析表明,GNP 主要激活控制炎症和凋亡途径的基因,而 GO 仅显示有限的炎症反应。有趣的是,这两种 GRM 都会影响与抗原加工和呈递相关的基因的表达,此外,GO 激活了 MDM 中中性粒细胞激活、脱颗粒和免疫的途径。总的来说,这项研究为未来在更先进的模型系统中评估 GRM 的安全性提供了广泛的潜在毒性机制资源,以验证在人类巨噬细胞中观察到的基因表达变化是否会对人类健康产生长期影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号