Energetic Materials Frontiers Pub Date : 2023-03-30 , DOI: 10.1016/j.enmf.2022.12.005 Zhi Wang , Li-han Fei , Hong-lei Xia , Yun-he Jin , Qing-hua Zhang
|
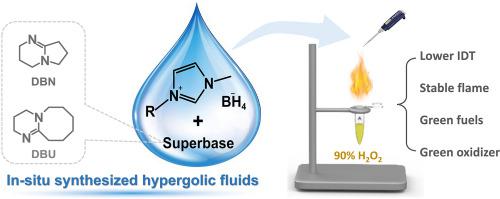
This study prepared a series of novel hypergolic fluids based on borohydride ionic liquids and organic superbase using an in situ synthetic method. In these hypergolic fluids, ionic liquids in 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) acted as triggers for the self-ignition of DBN and DBU upon contact with high-concentration hydrogen peroxide (H2O2). These hypergolic fluids had high densities (>1.000 g cm−3), low viscosities (as low as 34.03 cP), and acceptable ignition delay times (IDT). The ignition processes of the hypergolic fluids with 90% H2O2 as an oxidizer were first investigated in this study, and they differed from the previously reported ignition phenomena. Different from the case with white fuming acid (WFNA) as an oxidizer, the ignition processes of hypergolic fluids with 90% H2O2 as an oxidizer did not exhibit secondary rebound and splashing and formed a homogeneous mixed layer when the droplets were in contact with 90% H2O2. The different ignition processes significantly influenced the properties of hypergolic fluids. Compared with the hypergolic fluids with WFNA as an oxidizer, those with 90% H2O2 as an oxidizer showed a shorter IDT (IDTmin[90% H2O2]=28.3 ms, IDTmin[WFNA]=126 ms) and formed stable flames without secondary combustion. These results demonstrate that the in-situ synthesized fuels in this study hold great promise as green fuels in hypergolic propulsion systems.
中文翻译:

有机超碱介导合成硼氢化物离子液体作为新型复合自燃燃料
本研究采用原位合成方法制备了一系列基于硼氢化物离子液体和有机超强碱的新型自溶流体。在这些自液体中,1,5-二氮杂双环[4.3.0]non-5-ene (DBN) 和 1,8-二氮杂双环[5.4.0]undec-7-ene (DBU) 中的离子液体充当了DBN和DBU与高浓度过氧化氢(H 2 O 2 )接触时会自燃。这些自燃流体具有高密度(>1.000 gcm −3)、低粘度(低至 34.03 cP)和可接受的点火延迟时间 (IDT)。90% H 2 O 2自燃流体的着火过程本研究首次研究了作为氧化剂的燃烧现象,它们与之前报道的点火现象不同。与以白发烟酸(WFNA)为氧化剂的情况不同,以90%H 2 O 2为氧化剂的自燃液体的着火过程没有出现二次反弹和飞溅,并且在液滴接触时形成均匀的混合层90% H 2 O 2。不同的点火过程显着影响自燃流体的性质。与以 WFNA 作为氧化剂的自高液体相比,以 90% H 2 O 2作为氧化剂的自液体表现出更短的 IDT (IDT min[90% H2O2]= 28.3 ms,IDTmin[WFNA]= 126ms)并形成稳定火焰,无需二次燃烧。这些结果表明,本研究中的原位合成燃料作为自燃推进系统中的绿色燃料具有广阔的前景。



























 京公网安备 11010802027423号
京公网安备 11010802027423号