当前位置:
X-MOL 学术
›
WIREs Nanomed. Nanobiotechnol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organoids technology for advancing the clinical translation of cancer nanomedicine
WIREs Nanomedicine and Nanobiotechnology ( IF 8.6 ) Pub Date : 2023-04-23 , DOI: 10.1002/wnan.1892 Dong-Kun Zhao 1 , Jie Liang 1, 2 , Xiao-Yi Huang 1, 3 , Song Shen 1, 3 , Jun Wang 1, 4, 5
WIREs Nanomedicine and Nanobiotechnology ( IF 8.6 ) Pub Date : 2023-04-23 , DOI: 10.1002/wnan.1892 Dong-Kun Zhao 1 , Jie Liang 1, 2 , Xiao-Yi Huang 1, 3 , Song Shen 1, 3 , Jun Wang 1, 4, 5
Affiliation
|
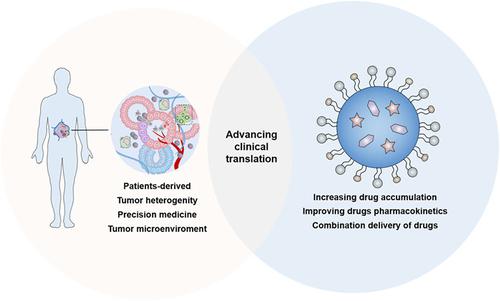
The past decades have witnessed the rapid development and widespread application of nanomedicines in cancer treatment; however, the clinical translation of experimental findings has been low, as evidenced by the low percentage of commercialized nanomedicines. Incomplete understanding of nanomedicine-tumor interactions and inappropriate evaluation models are two important challenges limiting the clinical translation of cancer nanomedicines. Currently, nanomedicine-tumor interaction and therapeutic effects are mainly investigated using cell lines or mouse models, which do not recapitulate the complex tumor microenvironment in human patients. Thus, information obtained from cell lines and mouse models cannot provide adequate guidance for the rational redesign of nanomedicine. Compared with other preclinical models, tumor organoids constructed from patient-derived tumor tissues are superior in retaining the key histopathological, genetic, and phenotypic features of the parent tumor. We speculate that organoid technology would help elucidate nanomedicine-tumor interaction in the tumor microenvironment and guide the design of nanomedicine, making it a reliable tool to accurately predict drug responses in patients with cancer. This review highlighted the advantages of drug delivery systems in cancer treatment, challenges limiting the clinical translation of antitumor nanomedicines, and potential application of patient-derived organoids (PDO) in nanomedicine. We propose that combining organoids and nanotechnology would facilitate the development of safe and effective cancer nanomedicines and accelerate their clinical application. This review discussed the potential translational value of integrative research using organoids and cancer nanomedicine.
中文翻译:

类器官技术促进癌症纳米医学的临床转化
过去几十年见证了纳米药物在癌症治疗中的快速发展和广泛应用;然而,实验结果的临床转化率很低,商业化纳米药物的比例较低就证明了这一点。对纳米药物与肿瘤相互作用的不完全理解和不适当的评估模型是限制癌症纳米药物临床转化的两个重要挑战。目前,纳米药物与肿瘤的相互作用和治疗效果主要使用细胞系或小鼠模型进行研究,这些模型并不能重现人类患者复杂的肿瘤微环境。因此,从细胞系和小鼠模型获得的信息不能为纳米医学的合理重新设计提供足够的指导。与其他临床前模型相比,由患者来源的肿瘤组织构建的肿瘤类器官在保留母体肿瘤的关键组织病理学、遗传和表型特征方面具有优势。我们推测类器官技术将有助于阐明肿瘤微环境中纳米药物与肿瘤的相互作用,并指导纳米药物的设计,使其成为准确预测癌症患者药物反应的可靠工具。这篇综述强调了药物输送系统在癌症治疗中的优势、限制抗肿瘤纳米药物临床转化的挑战以及患者源性类器官(PDO)在纳米医学中的潜在应用。我们认为,类器官和纳米技术的结合将有助于开发安全有效的癌症纳米药物并加速其临床应用。这篇综述讨论了使用类器官和癌症纳米医学进行综合研究的潜在转化价值。
更新日期:2023-04-23
中文翻译:

类器官技术促进癌症纳米医学的临床转化
过去几十年见证了纳米药物在癌症治疗中的快速发展和广泛应用;然而,实验结果的临床转化率很低,商业化纳米药物的比例较低就证明了这一点。对纳米药物与肿瘤相互作用的不完全理解和不适当的评估模型是限制癌症纳米药物临床转化的两个重要挑战。目前,纳米药物与肿瘤的相互作用和治疗效果主要使用细胞系或小鼠模型进行研究,这些模型并不能重现人类患者复杂的肿瘤微环境。因此,从细胞系和小鼠模型获得的信息不能为纳米医学的合理重新设计提供足够的指导。与其他临床前模型相比,由患者来源的肿瘤组织构建的肿瘤类器官在保留母体肿瘤的关键组织病理学、遗传和表型特征方面具有优势。我们推测类器官技术将有助于阐明肿瘤微环境中纳米药物与肿瘤的相互作用,并指导纳米药物的设计,使其成为准确预测癌症患者药物反应的可靠工具。这篇综述强调了药物输送系统在癌症治疗中的优势、限制抗肿瘤纳米药物临床转化的挑战以及患者源性类器官(PDO)在纳米医学中的潜在应用。我们认为,类器官和纳米技术的结合将有助于开发安全有效的癌症纳米药物并加速其临床应用。这篇综述讨论了使用类器官和癌症纳米医学进行综合研究的潜在转化价值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号