当前位置:
X-MOL 学术
›
Biopolymers
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New insights from modelling studies and molecular dynamics simulations of the DIS5-S6 extracellular linker of the skeletal muscle sodium channel NaV1.4
Biopolymers ( IF 2.9 ) Pub Date : 2023-05-31 , DOI: 10.1002/bip.23540 Anna Robinson 1 , Elaine Tao 2 , Teresa Neeman 3 , Benjamin Kaehler 4 , Ben Corry 2
Biopolymers ( IF 2.9 ) Pub Date : 2023-05-31 , DOI: 10.1002/bip.23540 Anna Robinson 1 , Elaine Tao 2 , Teresa Neeman 3 , Benjamin Kaehler 4 , Ben Corry 2
Affiliation
|
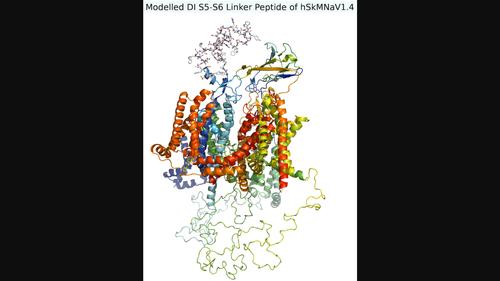
In the CryoEM-structure of the hSkMNaV1.4 ion channel (PDB:6AGF), the 59-residue DIS5-S6 linker peptide was omitted due to absence of electron density. This peptide is intriguing – comprised of unique sequence and found only in mammalian skeletal muscle sodium ion channels. To probe potential physiological and evolutionary significance, we constructed an homology model of the complete hSkMNaV1.4 channel. Rather than a flexible random coil potentiating drift across the channel, the linker folds into a compact configuration through self-assembling secondary structural elements. Analogous sequences from 48 mammalian organisms show hypervariability with between 40% and 100% sequence similarity. To investigate structural implications, sequences from 14 representative organisms were additionally modelled. All showed highly conserved N-and C-terminal residues closely superimposed, suggesting a critical functional role. An optimally located asparagine residue within the conserved region was investigated for N-linked glycosylation and MD simulations carried out. Results suggest a complex glycan added at this site in the linker may form electrostatic interactions with the DIV voltage sensing domain and be mechanistically involved in channel gating. The relationship of unique sequence, compact configuration, potential glycosylation and MD simulations are discussed relative to SkMNaV1.4 structure and function.
中文翻译:

骨骼肌钠通道 NaV1.4 DIS5-S6 细胞外连接子的建模研究和分子动力学模拟的新见解
在hSkMNaV1.4离子通道 (PDB:6AGF)的 CryoEM 结构中,由于缺乏电子密度,省略了59 个残基 DI S5-S6连接肽。这种肽很有趣——由独特的序列组成,仅在哺乳动物骨骼肌钠离子通道中发现。为了探讨潜在的生理和进化意义,我们构建了完整hSkMNaV1.4的同源模型渠道。连接体不是通过灵活的随机卷曲来增强跨通道的漂移,而是通过自组装的二级结构元件折叠成紧凑的结构。来自 48 种哺乳动物的类似序列显示出高度变异性,序列相似性在 40% 到 100% 之间。为了研究结构意义,还对 14 种代表性生物体的序列进行了建模。所有这些都显示出高度保守的 N 端和 C 端残基紧密重叠,表明其具有关键的功能作用。研究了保守区域内最佳位置的天冬酰胺残基的 N 连接糖基化并进行了 MD 模拟。结果表明,在连接子的该位点添加的复杂聚糖可能与 DIV 电压传感域形成静电相互作用,并机械地参与通道门控。SkMNaV1.4结构和功能。
更新日期:2023-05-31
中文翻译:

骨骼肌钠通道 NaV1.4 DIS5-S6 细胞外连接子的建模研究和分子动力学模拟的新见解
在hSkMNaV1.4离子通道 (PDB:6AGF)的 CryoEM 结构中,由于缺乏电子密度,省略了59 个残基 DI S5-S6连接肽。这种肽很有趣——由独特的序列组成,仅在哺乳动物骨骼肌钠离子通道中发现。为了探讨潜在的生理和进化意义,我们构建了完整hSkMNaV1.4的同源模型渠道。连接体不是通过灵活的随机卷曲来增强跨通道的漂移,而是通过自组装的二级结构元件折叠成紧凑的结构。来自 48 种哺乳动物的类似序列显示出高度变异性,序列相似性在 40% 到 100% 之间。为了研究结构意义,还对 14 种代表性生物体的序列进行了建模。所有这些都显示出高度保守的 N 端和 C 端残基紧密重叠,表明其具有关键的功能作用。研究了保守区域内最佳位置的天冬酰胺残基的 N 连接糖基化并进行了 MD 模拟。结果表明,在连接子的该位点添加的复杂聚糖可能与 DIV 电压传感域形成静电相互作用,并机械地参与通道门控。SkMNaV1.4结构和功能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号