当前位置:
X-MOL 学术
›
J. Label. Comp. Radiopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development and physicochemical characterization of a biodegradable microspheres formulation loaded with samarium-153 and doxorubicin for chemo-radioembolization of liver tumours
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 1.8 ) Pub Date : 2023-06-07 , DOI: 10.1002/jlcr.4046 Asseel Hisham Alregib 1 , Hun Yee Tan 2 , Yin How Wong 1, 3 , Azahari Kasbollah 4 , Eng Hwa Wong 1, 3 , Basri Johan Jeet Abdullah 1, 2, 5 , Alan Christopher Perkins 6 , Chai Hong Yeong 1, 3
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 1.8 ) Pub Date : 2023-06-07 , DOI: 10.1002/jlcr.4046 Asseel Hisham Alregib 1 , Hun Yee Tan 2 , Yin How Wong 1, 3 , Azahari Kasbollah 4 , Eng Hwa Wong 1, 3 , Basri Johan Jeet Abdullah 1, 2, 5 , Alan Christopher Perkins 6 , Chai Hong Yeong 1, 3
Affiliation
|
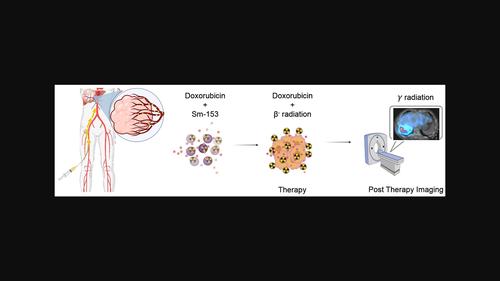
Transarterial chemoembolization (TACE) and transarterial radioembolization (TARE) are promising treatments for unresectable liver tumours. Some recent studies suggested that combining TACE and TARE in one treatment course might improve treatment efficacy through synergistic cytotoxicity effects. Nonetheless, current formulations do not facilitate a combination of chemo- and radio-embolic agents in one delivery system. Therefore, this study aimed to synthesise a hybrid biodegradable microsphere loaded with both radioactive agent, samarium-153 (153Sm) and chemotherapeutic drug, doxorubicin (Dox) for potential radio-chemoembolization of advanced liver tumours. 152Sm and Dox-loaded polyhydroxybutyrate-co-3-hydroxyvalerate (PHBV) microspheres were prepared using water-in-oil-in-water solvent evaporation method. The microspheres were then sent for neutron activation in a neutron flux of 2 × 1012 n/cm2/s. The physicochemical properties, radioactivity, radionuclide purity, 153Sm retention efficiency, and Dox release profile of the Dox-153Sm-PHBV microspheres were analysed. In addition, in vitro cytotoxicity of the formulation was tested using MTT assay on HepG2 cell line at 24 and 72 h. The mean diameter of the Dox-153Sm-PHBV microspheres was 30.08 ± 2.79 μm. The specific radioactivity was 8.68 ± 0.17 GBq/g, or 177.69 Bq per microsphere. The 153Sm retention efficiency was more than 99%, tested in phosphate-buffered saline (PBS) and human blood plasma over 26 days. The cumulative release of Dox from the microspheres after 41 days was 65.21 ± 1.96% and 29.96 ± 0.03% in PBS solution of pH 7.4 and pH 5.5, respectively. The Dox-153Sm-PHBV microspheres achieved a greater in vitro cytotoxicity effect on HepG2 cells (85.73 ± 3.63%) than 153Sm-PHBV (70.03 ± 5.61%) and Dox-PHBV (74.06 ± 0.78%) microspheres at 300 μg/mL at 72 h. In conclusion, a novel biodegradable microspheres formulation loaded with chemotherapeutic drug (Dox) and radioactive agent (153Sm) was successfully developed in this study. The formulation fulfilled all the desired physicochemical properties of a chemo-radioembolic agent and achieved better in vitro cytotoxicity on HepG2 cells. Further investigations are needed to evaluate the biosafety, radiation dosimetry, and synergetic anticancer properties of the formulation.
中文翻译:

用于肝肿瘤化疗放射栓塞的载有钐153和阿霉素的可生物降解微球制剂的开发和理化表征
经动脉化疗栓塞(TACE)和经动脉放射栓塞(TARE)是治疗不可切除的肝脏肿瘤的有前途的治疗方法。最近的一些研究表明,在一个疗程中结合 TACE 和 TARE 可能会通过协同细胞毒性作用来提高治疗效果。尽管如此,目前的制剂并不能促进化学栓塞剂和放射栓塞剂在一个递送系统中的组合。因此,本研究旨在合成一种载有放射性试剂钐153(153 Sm)和化疗药物阿霉素(Dox)的混合可生物降解微球,用于晚期肝脏肿瘤的潜在放射化学栓塞。采用水包油包水溶剂蒸发法制备了负载152 Sm和Dox的聚羟基丁酸酯-co-3-羟基戊酸酯(PHBV)微球。然后将微球以2×10 12 n/cm 2 /s的中子通量送去中子活化。分析了Dox- 153 Sm-PHBV 微球的理化性质、放射性、放射性核素纯度、153 Sm 保留效率和Dox 释放曲线。此外,在 24 小时和 72 小时时,使用 MTT 法对 HepG2 细胞系测试了制剂的体外细胞毒性。Dox- 153 Sm-PHBV 微球的平均直径为 30.08 ± 2.79 μm。比放射性为 8.68 ± 0.17 GBq/g,或每个微球 177.69 Bq。在磷酸盐缓冲盐水 (PBS) 和人血浆中经过 26 天的测试,153 Sm保留效率超过 99%。41 天后,在 pH 7.4 和 5.5 的 PBS 溶液中,Dox 从微球中的累积释放量分别为 65.21 ± 1.96% 和 29.96 ± 0.03%。300 μg /dox- 153 Sm-PHBV 微球对 HepG2 细胞的体外细胞毒性作用(85.73 ± 3.63%)优于153 Sm-PHBV(70.03 ± 5.61%)和 Dox-PHBV(74.06 ± 0.78%)微球。 72 小时时的毫升数。总之,本研究成功开发了一种负载化疗药物(Dox)和放射性试剂( 153 Sm)的新型可生物降解微球制剂。该制剂满足了化学放射栓塞剂的所有所需理化特性,并对 HepG2 细胞实现了更好的体外细胞毒性。需要进一步研究来评估该制剂的生物安全性、辐射剂量测定和协同抗癌特性。
更新日期:2023-06-07
中文翻译:

用于肝肿瘤化疗放射栓塞的载有钐153和阿霉素的可生物降解微球制剂的开发和理化表征
经动脉化疗栓塞(TACE)和经动脉放射栓塞(TARE)是治疗不可切除的肝脏肿瘤的有前途的治疗方法。最近的一些研究表明,在一个疗程中结合 TACE 和 TARE 可能会通过协同细胞毒性作用来提高治疗效果。尽管如此,目前的制剂并不能促进化学栓塞剂和放射栓塞剂在一个递送系统中的组合。因此,本研究旨在合成一种载有放射性试剂钐153(153 Sm)和化疗药物阿霉素(Dox)的混合可生物降解微球,用于晚期肝脏肿瘤的潜在放射化学栓塞。采用水包油包水溶剂蒸发法制备了负载152 Sm和Dox的聚羟基丁酸酯-co-3-羟基戊酸酯(PHBV)微球。然后将微球以2×10 12 n/cm 2 /s的中子通量送去中子活化。分析了Dox- 153 Sm-PHBV 微球的理化性质、放射性、放射性核素纯度、153 Sm 保留效率和Dox 释放曲线。此外,在 24 小时和 72 小时时,使用 MTT 法对 HepG2 细胞系测试了制剂的体外细胞毒性。Dox- 153 Sm-PHBV 微球的平均直径为 30.08 ± 2.79 μm。比放射性为 8.68 ± 0.17 GBq/g,或每个微球 177.69 Bq。在磷酸盐缓冲盐水 (PBS) 和人血浆中经过 26 天的测试,153 Sm保留效率超过 99%。41 天后,在 pH 7.4 和 5.5 的 PBS 溶液中,Dox 从微球中的累积释放量分别为 65.21 ± 1.96% 和 29.96 ± 0.03%。300 μg /dox- 153 Sm-PHBV 微球对 HepG2 细胞的体外细胞毒性作用(85.73 ± 3.63%)优于153 Sm-PHBV(70.03 ± 5.61%)和 Dox-PHBV(74.06 ± 0.78%)微球。 72 小时时的毫升数。总之,本研究成功开发了一种负载化疗药物(Dox)和放射性试剂( 153 Sm)的新型可生物降解微球制剂。该制剂满足了化学放射栓塞剂的所有所需理化特性,并对 HepG2 细胞实现了更好的体外细胞毒性。需要进一步研究来评估该制剂的生物安全性、辐射剂量测定和协同抗癌特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号