当前位置:
X-MOL 学术
›
Biopolymers
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient production of fluorophore-labeled CC chemokines for biophysical studies using recombinant enterokinase and recombinant sortase
Biopolymers ( IF 2.9 ) Pub Date : 2023-06-21 , DOI: 10.1002/bip.23557 Wenyan Guan 1 , Ning Zhang 2 , Arjan Bains 3 , Mourad Sadqi 4 , Cynthia M Dupureur 5 , Patricia J LiWang 6
Biopolymers ( IF 2.9 ) Pub Date : 2023-06-21 , DOI: 10.1002/bip.23557 Wenyan Guan 1 , Ning Zhang 2 , Arjan Bains 3 , Mourad Sadqi 4 , Cynthia M Dupureur 5 , Patricia J LiWang 6
Affiliation
|
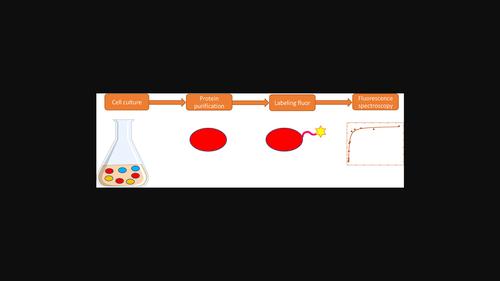
Chemokines are important immune system proteins, many of which mediate inflammation due to their function to activate and cause chemotaxis of leukocytes. An important anti-inflammatory strategy is therefore to bind and inhibit chemokines, which leads to the need for biophysical studies of chemokines as they bind various possible partners. Because a successful anti-chemokine drug should bind at low concentrations, techniques such as fluorescence anisotropy that can provide nanomolar signal detection are required. To allow fluorescence experiments to be carried out on chemokines, a method is described for the production of fluorescently labeled chemokines. First, a fusion-tagged chemokine is produced in Escherichia coli, then efficient cleavage of the N-terminal fusion partner is carried out with lab-produced enterokinase, followed by covalent modification with a fluorophore, mediated by the lab-produced sortase enzyme. This overall process reduces the need for expensive commercial enzymatic reagents. Finally, we utilize the product, vMIP-fluor, in binding studies with the chemokine binding protein vCCI, which has great potential as an anti-inflammatory therapeutic, showing a binding constant for vCCI:vMIP-fluor of 0.37 ± 0.006 nM. We also show how a single modified chemokine homolog (vMIP-fluor) can be used in competition assays with other chemokines and we report a Kd for vCCI:CCL17 of 14 μM. This work demonstrates an efficient method of production and fluorescent labeling of chemokines for study across a broad range of concentrations.
中文翻译:

使用重组肠激酶和重组分选酶高效生产用于生物物理研究的荧光团标记的 CC 趋化因子
趋化因子是重要的免疫系统蛋白,其中许多由于其激活和引起白细胞趋化性的功能而介导炎症。因此,一个重要的抗炎策略是结合和抑制趋化因子,这导致需要对趋化因子进行生物物理学研究,因为它们结合各种可能的伙伴。由于成功的抗趋化因子药物应在低浓度下结合,因此需要能够提供纳摩尔级信号检测的荧光各向异性等技术。为了能够对趋化因子进行荧光实验,描述了一种用于产生荧光标记趋化因子的方法。首先,在大肠杆菌中产生融合标记的趋化因子,然后使用实验室生产的肠激酶对 N 末端融合伴侣进行有效切割,然后在实验室生产的分选酶的介导下用荧光团进行共价修饰。这个整体过程减少了对昂贵的商业酶试剂的需求。最后,我们在与趋化因子结合蛋白 vCCI 的结合研究中利用产品 vMIP-flor,该蛋白作为抗炎治疗剂具有巨大潜力,显示 vCCI:vMIP-flor 的结合常数为 0.37 ± 0.006 nM。我们还展示了如何将单一修饰的趋化因子同系物 (vMIP-flor) 用于与其他趋化因子的竞争测定中,并且我们报告vCCI:CCL17 的K d为 14 μM。这项工作展示了一种有效的趋化因子生产和荧光标记方法,可用于广泛浓度范围的研究。
更新日期:2023-06-21
中文翻译:

使用重组肠激酶和重组分选酶高效生产用于生物物理研究的荧光团标记的 CC 趋化因子
趋化因子是重要的免疫系统蛋白,其中许多由于其激活和引起白细胞趋化性的功能而介导炎症。因此,一个重要的抗炎策略是结合和抑制趋化因子,这导致需要对趋化因子进行生物物理学研究,因为它们结合各种可能的伙伴。由于成功的抗趋化因子药物应在低浓度下结合,因此需要能够提供纳摩尔级信号检测的荧光各向异性等技术。为了能够对趋化因子进行荧光实验,描述了一种用于产生荧光标记趋化因子的方法。首先,在大肠杆菌中产生融合标记的趋化因子,然后使用实验室生产的肠激酶对 N 末端融合伴侣进行有效切割,然后在实验室生产的分选酶的介导下用荧光团进行共价修饰。这个整体过程减少了对昂贵的商业酶试剂的需求。最后,我们在与趋化因子结合蛋白 vCCI 的结合研究中利用产品 vMIP-flor,该蛋白作为抗炎治疗剂具有巨大潜力,显示 vCCI:vMIP-flor 的结合常数为 0.37 ± 0.006 nM。我们还展示了如何将单一修饰的趋化因子同系物 (vMIP-flor) 用于与其他趋化因子的竞争测定中,并且我们报告vCCI:CCL17 的K d为 14 μM。这项工作展示了一种有效的趋化因子生产和荧光标记方法,可用于广泛浓度范围的研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号