Journal of Structural Biology ( IF 3 ) Pub Date : 2023-08-09 , DOI: 10.1016/j.jsb.2023.108011 Abraham Takkouche 1 , Xinru Qiu 2 , Mayya Sedova 3 , Lukasz Jaroszewski 3 , Adam Godzik 3
|
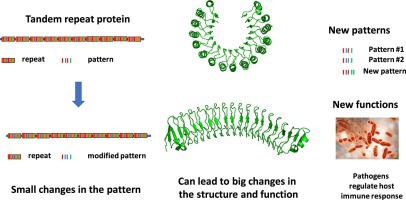
Leucine Rich Repeat (LRR) domains, are present in hundreds of thousands of proteins across all kingdoms of life and are typically involved in protein–protein interactions and ligand recognition. LRR domains are classified into eight classes and when examined in three dimensions seven, of them form curved solenoid-like super-helices, also described as toruses, with a beta sheet on the concave (inside) and stacked alpha-helices on the convex (outside) of the torus. Here we present an overview of the least characterized 8th class of LRR proteins, the TpLRR-like LRRs, named after the Treponema pallidum protein Tp0225. Proteins from the TpLRR class differ from the proteins in all other known LRR classes by having a flipped curvature, with the beta sheet on the convex side of the torus and irregular secondary structure instead of helices on the opposite, now concave site. TpLRR proteins also present highly divergent sequence pattern of individual repeats and can associate with specific types of additional domains. Several of the characterized proteins from this class, specifically the BspA-like proteins, were found in human bacterial and protozoan pathogens, playing an important role in the interactions between the pathogens and the host immune system. In this paper we surveyed all existing experimental structures and selected AlphaFold models of the best-known proteins containing this class of LRR repeats, analyzing the relation between the pattern of conserved residues, specific structural features and functions of these proteins.
中文翻译:

TpLRR/BspA 样 LRR 蛋白的异常结构和功能特征
富含亮氨酸重复序列 (LRR) 结构域存在于生命各个领域的数十万种蛋白质中,通常参与蛋白质-蛋白质相互作用和配体识别。LRR 结构域分为八类,当在三个维度七中进行检查时,它们形成弯曲的螺线管状超级螺旋,也称为圆环,凹面(内部)有 β 片层,凸面有堆叠的 α 螺旋(外部)的环面。在这里,我们概述了特征最少的第 8 类 LRR 蛋白,即 TpLRR 样 LRR,以梅毒螺旋体蛋白 Tp0225 命名。TpLRR 类蛋白质与所有其他已知 LRR 类蛋白质的不同之处在于具有翻转的曲率,β 片层位于环面的凸侧,并且不规则的二级结构而不是位于相反的凹位点上的螺旋。TpLRR 蛋白还呈现出高度不同的个体重复序列模式,并且可以与特定类型的附加结构域相关联。在人类细菌和原生动物病原体中发现了此类的几种特征蛋白,特别是 BspA 样蛋白,在病原体与宿主免疫系统之间的相互作用中发挥着重要作用。在本文中,我们调查了所有现有的实验结构,并选择了包含此类LRR重复的最著名蛋白质的AlphaFold模型,分析了这些蛋白质的保守残基模式、具体结构特征和功能之间的关系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号