Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2023-10-04 , DOI: 10.1016/j.yjmcc.2023.09.008 Valentin Burkart 1 , Kathrin Kowalski 1 , Alina Disch 1 , Denise Hilfiker-Kleiner 2 , Sean Lal 3 , Cristobal Dos Remedios 4 , Andreas Perrot 5 , Andre Zeug 6 , Evgeni Ponimaskin 6 , Maike Kosanke 7 , Oliver Dittrich-Breiholz 7 , Theresia Kraft 1 , Judith Montag 1
|
Hypertrophic cardiomyopathy (HCM) is the most prevalent inherited cardiac disease. Up to 40% of cases are associated with heterozygous mutations in myosin binding protein C (cMyBP-C, MYBPC3). Most of these mutations lead to premature termination codons (PTC) and patients show reduction of functional cMyBP-C. This so-called haploinsufficiency most likely contributes to disease development.
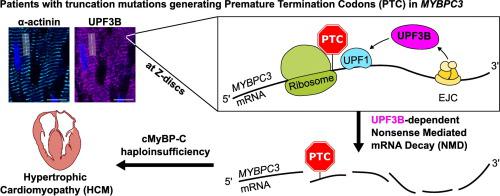
We analyzed mechanisms underlying haploinsufficiency using cardiac tissue from HCM-patients with truncation mutations in MYBPC3 (MYBPC3trunc). We compared transcriptional activity, mRNA and protein expression to donor controls. To differentiate between HCM-specific and general hypertrophy-induced mechanisms we used patients with left ventricular hypertrophy due to aortic stenosis (AS) as an additional control. We show that cMyBP-C haploinsufficiency starts at the mRNA level, despite hypertrophy-induced increased transcriptional activity. Gene set enrichment analysis (GSEA) of RNA-sequencing data revealed an increased expression of NMD-components. Among them, Up-frameshift protein UPF3B, a regulator of NMD was upregulated in MYBPC3trunc patients and not in AS-patients. Strikingly, we show that in sarcomeres UPF3B but not UPF1 and UPF2 are localized to the Z-discs, the presumed location of sarcomeric protein translation. Our data suggest that cMyBP-C haploinsufficiency in HCM-patients is established by UPF3B-dependent NMD during the initial translation round at the Z-disc.
中文翻译:

无义介导的衰变因子 UPF3B 与肥厚型心肌病患者的 cMyBP-C 单倍体不足相关
肥厚型心肌病(HCM)是最常见的遗传性心脏病。高达 40% 的病例与肌球蛋白结合蛋白 C(cMyBP-C、 MYBPC3 )杂合突变有关。大多数这些突变会导致提前终止密码子 (PTC),并且患者表现出功能性 cMyBP-C 的减少。这种所谓的单倍体不足很可能导致疾病的发展。
我们使用MYBPC3截短突变( MYBPC3 trunc ) 的 HCM 患者的心脏组织分析了单倍体不足的机制。我们将转录活性、mRNA 和蛋白质表达与供体对照进行了比较。为了区分 HCM 特异性和一般肥厚诱发机制,我们使用因主动脉瓣狭窄 (AS) 导致左心室肥厚的患者作为额外对照。我们表明,尽管肥大诱导转录活性增加,但 cMyBP-C 单倍体不足始于 mRNA 水平。RNA 测序数据的基因集富集分析 (GSEA) 显示 NMD 成分的表达增加。其中,上移码蛋白 UPF3B(NMD 的调节因子)在MYBPC3 trunc患者中表达上调,而在 AS 患者中则没有上调。引人注目的是,我们发现在肌节中 UPF3B 而不是 UPF1 和 UPF2 定位于Z盘,这是肌节蛋白翻译的推测位置。我们的数据表明,HCM 患者中的 cMyBP-C 单倍体不足是由 UPF3B 依赖性 NMD 在Z盘的初始平移过程中建立的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号