Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2023-10-05 , DOI: 10.1016/j.yjmcc.2023.10.004 Roman Y Medvedev 1 , Daniel G P Turner 1 , Frank C DeGuire 1 , Vladislav Leonov 1 , Di Lang 2 , Julia Gorelik 3 , Francisco J Alvarado 1 , Vladimir E Bondarenko 4 , Alexey V Glukhov 1
|
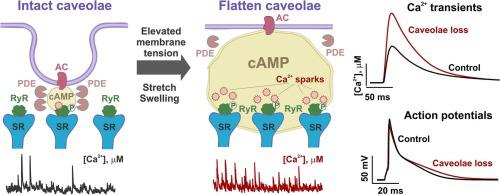
Caveolae are tiny invaginations in the sarcolemma that buffer extra membrane and contribute to mechanical regulation of cellular function. While the role of caveolae in membrane mechanosensation has been studied predominantly in non-cardiomyocyte cells, caveolae contribution to cardiac mechanotransduction remains elusive. Here, we studied the role of caveolae in the regulation of Ca2+ signaling in atrial cardiomyocytes. In Langendorff-perfused mouse hearts, atrial pressure/volume overload stretched atrial myocytes and decreased caveolae density. In isolated cells, caveolae were disrupted through hypotonic challenge that induced a temporal (<10 min) augmentation of Ca2+ transients and caused a rise in Ca2+ spark activity. Similar changes in Ca2+ signaling were observed after chemical (methyl-β-cyclodextrin) and genetic ablation of caveolae in cardiac-specific conditional caveolin-3 knock-out mice. Acute disruption of caveolae, both mechanical and chemical, led to the elevation of cAMP level in the cell interior, and cAMP-mediated augmentation of protein kinase A (PKA)-phosphorylated ryanodine receptors (at Ser2030 and Ser2808). Caveolae-mediated stimulatory effects on Ca2+ signaling were abolished via inhibition of cAMP production by adenyl cyclase antagonists MDL12330 and SQ22536, or reduction of PKA activity by H-89. A compartmentalized mathematical model of mouse atrial myocytes linked the observed changes to a microdomain-specific decrease in phosphodiesterase activity, which disrupted cAMP signaling and augmented PKA activity. Our findings add a new dimension to cardiac mechanobiology and highlight caveolae-associated cAMP/PKA-mediated phosphorylation of Ca2+ handling proteins as a novel component of mechano-chemical feedback in atrial myocytes.
中文翻译:

小鼠心房肌细胞中小窝相关的 cAMP/Ca2+ 介导的机械化学信号转导
小凹是肌膜中的微小内陷,可缓冲额外的膜并有助于细胞功能的机械调节。虽然小窝在膜机械感觉中的作用主要在非心肌细胞中进行研究,但小窝对心脏机械转导的贡献仍然难以捉摸。在这里,我们研究了小窝在心房心肌细胞 Ca 2+信号传导调节中的作用。在 Langendorff 灌注的小鼠心脏中,心房压力/容量超负荷会拉伸心房肌细胞并降低小窝密度。在分离的细胞中,通过低渗激发破坏小凹,诱导 Ca 2+瞬变暂时(<10 分钟)增强,并导致 Ca 2+火花活性增加。在心脏特异性条件性小窝蛋白 3 敲除小鼠中,化学(甲基-β-环糊精)和基因消融小窝后,观察到Ca 2+信号传导的类似变化。小穴的急性破坏,包括机械和化学方面的破坏,导致细胞内部cAMP水平升高,以及 cAMP 介导的蛋白激酶 A (PKA) 磷酸化兰尼碱受体(Ser 2030和 Ser 2808)的增强。通过腺苷酸环化酶拮抗剂 MDL12330 和 SQ22536 抑制 cAMP 产生,或通过 H-89 降低 PKA 活性,消除了小凹介导的 Ca 2+信号传导刺激作用小鼠心房肌细胞的区室化数学模型将观察到的变化与磷酸二酯酶活性的微域特异性降低联系起来,这破坏了 cAMP 信号传导并增强了 PKA 活性。我们的研究结果为心脏机械生物学增加了一个新的维度,并强调了小窝相关的 cAMP/PKA 介导的 Ca 2+处理蛋白磷酸化是心房肌细胞机械化学反馈的新组成部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号