American Journal of Human Genetics ( IF 9.8 ) Pub Date : 2023-10-05 , DOI: 10.1016/j.ajhg.2023.09.004 Xi Shi 1 , Congyi Lu 2 , Alba Corman 2 , Alexandra Nikish 3 , Yang Zhou 4 , Randy J Platt 5 , Ivan Iossifov 6 , Feng Zhang 3 , Jen Q Pan 7 , Neville E Sanjana 2
|
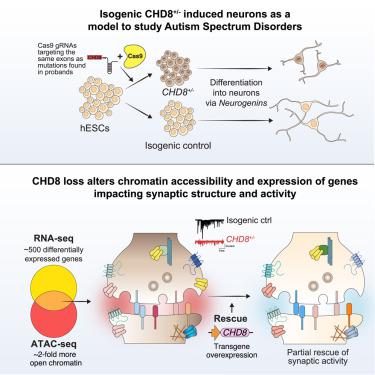
Whole-exome sequencing of autism spectrum disorder (ASD) probands and unaffected family members has identified many genes harboring de novo variants suspected to play a causal role in the disorder. Of these, chromodomain helicase DNA-binding protein 8 (CHD8) is the most recurrently mutated. Despite the prevalence of CHD8 mutations, we have little insight into how CHD8 loss affects genome organization or the functional consequences of these molecular alterations in neurons. Here, we engineered two isogenic human embryonic stem cell lines with CHD8 loss-of-function mutations and characterized differences in differentiated human cortical neurons. We identified hundreds of genes with altered expression, including many involved in neural development and excitatory synaptic transmission. Field recordings and single-cell electrophysiology revealed a 3-fold decrease in firing rates and synaptic activity in CHD8+/− neurons, as well as a similar firing-rate deficit in primary cortical neurons from Chd8+/− mice. These alterations in neuron and synapse function can be reversed by CHD8 overexpression. Moreover, CHD8+/− neurons displayed a large increase in open chromatin across the genome, where the greatest change in compaction was near autism susceptibility candidate 2 (AUTS2), which encodes a transcriptional regulator implicated in ASD. Genes with changes in chromatin accessibility and expression in CHD8+/− neurons have significant overlap with genes mutated in probands for ASD, intellectual disability, and schizophrenia but not with genes mutated in healthy controls or other disease cohorts. Overall, this study characterizes key molecular alterations in genome structure and expression in CHD8+/− neurons and links these changes to impaired neuronal and synaptic function.
中文翻译:

自闭症相关基因 CHD8 的杂合缺失通过基因表达和染色质压缩的广泛变化损害突触功能
对自闭症谱系障碍 (ASD) 先证者和未受影响的家庭成员进行的全外显子组测序已鉴定出许多含有疑似在该疾病中起因果作用的新生变异的基因。其中,染色质结构域解旋酶 DNA 结合蛋白 8 ( CHD8 ) 是最常发生突变的。尽管CHD8突变很普遍,但我们对 CHD8 缺失如何影响基因组组织或神经元中这些分子改变的功能后果知之甚少。在这里,我们设计了两种具有CHD8功能丧失突变的同基因人类胚胎干细胞系,并表征了分化的人类皮质神经元的差异。我们鉴定了数百个表达发生改变的基因,其中许多与神经发育和兴奋性突触传递有关。现场记录和单细胞电生理学显示, CHD8 +/-神经元的放电率和突触活性降低了 3 倍, Chd8 +/-小鼠的初级皮质神经元也存在类似的放电率缺陷。CHD8过表达可以逆转神经元和突触功能的这些改变。此外,CHD8 +/-神经元在整个基因组中显示出开放染色质的大量增加,其中压缩变化最大的是自闭症易感性候选 2 ( AUTS2 ) 附近,它编码与 ASD 相关的转录调节因子。CHD8 +/-神经元中染色质可及性和表达发生变化的基因与自闭症谱系障碍、智力障碍和精神分裂症先证者中的突变基因有显着重叠,但与健康对照或其他疾病队列中的突变基因没有显着重叠。总体而言,这项研究描述了CHD8 +/-神经元基因组结构和表达的关键分子改变,并将这些变化与受损的神经元和突触功能联系起来。



























 京公网安备 11010802027423号
京公网安备 11010802027423号