Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Differentiation and identification of enantiomers by nuclear magnetic resonance spectroscopy with support of quantum mechanical computations
Chirality ( IF 2 ) Pub Date : 2023-10-12 , DOI: 10.1002/chir.23623 Artur Brzezicki 1, 2 , Piotr Garbacz 1
Chirality ( IF 2 ) Pub Date : 2023-10-12 , DOI: 10.1002/chir.23623 Artur Brzezicki 1, 2 , Piotr Garbacz 1
Affiliation
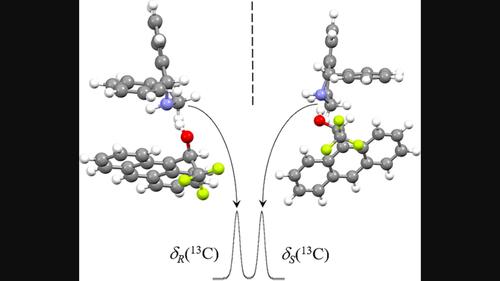
|
We report nuclear magnetic resonance studies of two chiral building blocks of solifenacin, phenyltetrahydroisoquinoline and quinuclidinol, in which chiral solvating agents, Mosher's acid, and Pirkle's alcohol were used for spectral discrimination between enantiomers of solifenacin constituents. Based on the constraints following from measurements of the nuclear Overhauser effect, structures of phenyltetrahydroisoquinoline and Pirkle's alcohol solvates were found. Next, shifts of nuclear magnetic resonance signals of phenyltetrahydroisoquinoline due to the application of Pirkle's alcohol were computed using density functional theory methods. The computed carbon-13 shifts reproduce those determined experimentally, allowing us to attribute the absolute configuration to phenyltetrahydroisoquinoline enantiomers without the need for the use of empirical rules.
中文翻译:

在量子力学计算的支持下通过核磁共振波谱区分和鉴定对映体
我们报告了索利那新的两种手性结构单元苯基四氢异喹啉和奎宁环醇的核磁共振研究,其中使用手性溶剂化剂莫舍酸和皮克尔醇来区分索利那新成分的对映体之间的光谱。基于核欧沃豪塞效应测量的限制,发现了苯基四氢异喹啉和皮克尔醇溶剂化物的结构。接下来,使用密度泛函理论方法计算由于皮尔克尔醇的应用而引起的苯基四氢异喹啉的核磁共振信号的变化。计算出的碳 13 位移再现了实验确定的位移,使我们能够将绝对构型归因于苯基四氢异喹啉对映体,而无需使用经验规则。
更新日期:2023-10-12
中文翻译:

在量子力学计算的支持下通过核磁共振波谱区分和鉴定对映体
我们报告了索利那新的两种手性结构单元苯基四氢异喹啉和奎宁环醇的核磁共振研究,其中使用手性溶剂化剂莫舍酸和皮克尔醇来区分索利那新成分的对映体之间的光谱。基于核欧沃豪塞效应测量的限制,发现了苯基四氢异喹啉和皮克尔醇溶剂化物的结构。接下来,使用密度泛函理论方法计算由于皮尔克尔醇的应用而引起的苯基四氢异喹啉的核磁共振信号的变化。计算出的碳 13 位移再现了实验确定的位移,使我们能够将绝对构型归因于苯基四氢异喹啉对映体,而无需使用经验规则。



























 京公网安备 11010802027423号
京公网安备 11010802027423号