当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Differences in heavy metal binding to cysteine-containing coiled-coil peptides
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2023-10-12 , DOI: 10.1002/psc.3549 Prianka Luther 1 , Aimee L Boyle 1
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2023-10-12 , DOI: 10.1002/psc.3549 Prianka Luther 1 , Aimee L Boyle 1
Affiliation
|
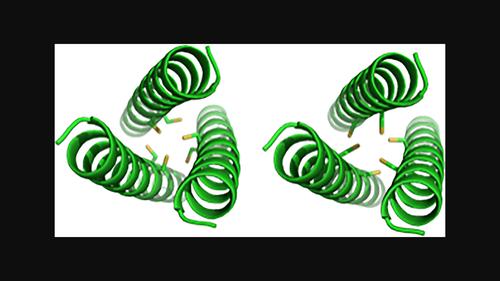
One third of all structurally characterised proteins contain a metal; however, the interplay between metal-binding and peptide/protein folding has yet to be fully elucidated. To better understand how metal binding affects peptide folding, a range of metals should be studied within a specific scaffold. To this end, we modified a histidine-containing coiled-coil peptide to create a cysteine-containing scaffold, named CX3C, which was designed to bind heavy metal ions. In addition, we generated a peptide named CX2C, which contains a binding site more commonly found in natural proteins. Using a combination of analytical techniques including circular dichroism (CD) spectroscopy, UV–Vis spectroscopy and size-exclusion chromatography coupled to multi-angle light scattering (SEC-MALS), we examined the differences in the metal-binding properties of the two peptides. Both peptides are largely unfolded in the apo state due to the disruption of the hydrophobic core by inclusion of the polar cysteine residues. However, this unfolding is overcome by the addition of Cd(II), Pb(II) and Hg(II), and helical assemblies are formed. Both peptides have differing affinities for these metal ions, a fact likely attributed to the differing sizes of the ions. We also show that the oligomerisation state of the peptide complexes and the coordination geometries of the metal ions differ between the two peptide scaffolds. These findings highlight that subtle changes in the primary structure of a peptide can have considerable implications for metal binding.
中文翻译:

重金属与含半胱氨酸卷曲螺旋肽结合的差异
所有具有结构特征的蛋白质中,有三分之一含有金属;然而,金属结合和肽/蛋白质折叠之间的相互作用尚未完全阐明。为了更好地了解金属结合如何影响肽折叠,应在特定支架内研究一系列金属。为此,我们修饰了含有组氨酸的卷曲螺旋肽,以创建含有半胱氨酸的支架,命名为 CX3C,旨在结合重金属离子。此外,我们还生成了一种名为 CX2C 的肽,它包含天然蛋白质中更常见的结合位点。结合使用圆二色性 (CD) 光谱、紫外-可见光谱和尺寸排阻色谱与多角度光散射 (SEC-MALS) 等分析技术,我们检查了两种肽的金属结合特性的差异。由于包含极性半胱氨酸残基破坏了疏水核心,两种肽大部分都以 apo 状态展开。然而,这种展开可以通过添加 Cd(II)、Pb(II) 和 Hg(II) 来克服,并形成螺旋组件。两种肽对这些金属离子具有不同的亲和力,这一事实可能归因于离子大小的不同。我们还表明,两种肽支架之间肽复合物的寡聚状态和金属离子的配位几何形状不同。这些发现强调,肽一级结构的细微变化可能对金属结合产生相当大的影响。
更新日期:2023-10-12
中文翻译:

重金属与含半胱氨酸卷曲螺旋肽结合的差异
所有具有结构特征的蛋白质中,有三分之一含有金属;然而,金属结合和肽/蛋白质折叠之间的相互作用尚未完全阐明。为了更好地了解金属结合如何影响肽折叠,应在特定支架内研究一系列金属。为此,我们修饰了含有组氨酸的卷曲螺旋肽,以创建含有半胱氨酸的支架,命名为 CX3C,旨在结合重金属离子。此外,我们还生成了一种名为 CX2C 的肽,它包含天然蛋白质中更常见的结合位点。结合使用圆二色性 (CD) 光谱、紫外-可见光谱和尺寸排阻色谱与多角度光散射 (SEC-MALS) 等分析技术,我们检查了两种肽的金属结合特性的差异。由于包含极性半胱氨酸残基破坏了疏水核心,两种肽大部分都以 apo 状态展开。然而,这种展开可以通过添加 Cd(II)、Pb(II) 和 Hg(II) 来克服,并形成螺旋组件。两种肽对这些金属离子具有不同的亲和力,这一事实可能归因于离子大小的不同。我们还表明,两种肽支架之间肽复合物的寡聚状态和金属离子的配位几何形状不同。这些发现强调,肽一级结构的细微变化可能对金属结合产生相当大的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号