当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dynemicin A Derivatives as Potential Cancer Chemotherapeutics by Mutasynthesis
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2023-10-21 , DOI: 10.1002/hlca.202300123 Craig A. Townsend 1 , Paramita Pal 2 , Jamie R. Alley 3 , Douglas R. Cohen 4
Helvetica Chimica Acta ( IF 1.8 ) Pub Date : 2023-10-21 , DOI: 10.1002/hlca.202300123 Craig A. Townsend 1 , Paramita Pal 2 , Jamie R. Alley 3 , Douglas R. Cohen 4
Affiliation
|
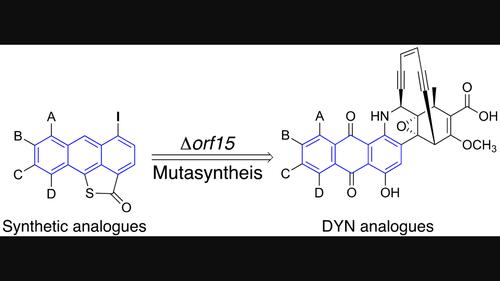
The enediyne antitumor antibiotics have remarkable structures and exhibit potent DNA cleavage properties that have inspired continued interest as cancer therapeutics. Their complex structures and high reactivity, however, pose formidable challenges to their production and development in the clinic. We report here proof-of-concept studies using a mutasynthesis strategy to combine chemical synthesis of select modifications to a key iodoanthracene-γ-thiolactone intermediate in the biosynthesis of dynemicin A and all other known anthraquinone-fused enediynes (AFEs). By chemical complementation of a mutant bacterial producer that is incapable of synthesizing this essential building block, we show that derivatives of dynemicin can be prepared substituted in the A-ring of the anthraquinone motif. In the absence of competition from native production of this intermediate, the most efficient utilization of these externally-supplied structural analogues for precursor-directed biosynthesis becomes possible. To achieve this goal, we describe the required Δorf15 blocked mutant and a general synthetic route to a library of iodoanthracene structural variants. Their successful incorporation opens the door to enhancing DNA binding and tuning the bioreductive activation of the modified enediynes for DNA cleavage.
中文翻译:

动力霉素 A 衍生物通过突变合成作为潜在的癌症化疗药物
烯二炔抗肿瘤抗生素具有非凡的结构,并表现出强大的 DNA 裂解特性,这激发了人们对癌症治疗的持续兴趣。然而,它们复杂的结构和高反应活性对其临床生产和开发提出了巨大的挑战。我们在这里报告了概念验证研究,使用变合成策略将化学合成与关键碘蒽-γ-硫内酯中间体的选择性修饰结合起来,用于动力霉素 A 和所有其他已知的蒽醌稠合烯二炔 (AFE) 的生物合成。通过对无法合成这种基本结构单元的突变细菌生产者进行化学互补,我们表明可以制备取代蒽醌基序 A 环的动力霉素衍生物。在没有来自该中间体的天然生产的竞争的情况下,最有效地利用这些外部提供的结构类似物进行前体定向的生物合成成为可能。为了实现这一目标,我们描述了所需的 Δ orf15阻断突变体以及碘蒽结构变体库的一般合成路线。它们的成功掺入为增强 DNA 结合和调节修饰烯二炔的生物还原活化以进行 DNA 切割打开了大门。
更新日期:2023-10-21
中文翻译:

动力霉素 A 衍生物通过突变合成作为潜在的癌症化疗药物
烯二炔抗肿瘤抗生素具有非凡的结构,并表现出强大的 DNA 裂解特性,这激发了人们对癌症治疗的持续兴趣。然而,它们复杂的结构和高反应活性对其临床生产和开发提出了巨大的挑战。我们在这里报告了概念验证研究,使用变合成策略将化学合成与关键碘蒽-γ-硫内酯中间体的选择性修饰结合起来,用于动力霉素 A 和所有其他已知的蒽醌稠合烯二炔 (AFE) 的生物合成。通过对无法合成这种基本结构单元的突变细菌生产者进行化学互补,我们表明可以制备取代蒽醌基序 A 环的动力霉素衍生物。在没有来自该中间体的天然生产的竞争的情况下,最有效地利用这些外部提供的结构类似物进行前体定向的生物合成成为可能。为了实现这一目标,我们描述了所需的 Δ orf15阻断突变体以及碘蒽结构变体库的一般合成路线。它们的成功掺入为增强 DNA 结合和调节修饰烯二炔的生物还原活化以进行 DNA 切割打开了大门。



























 京公网安备 11010802027423号
京公网安备 11010802027423号