Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2023-11-02 , DOI: 10.1016/j.yjmcc.2023.10.013 Colleen M Kelly 1 , Jody L Martin 2 , Molly Coseno 3 , Michael J Previs 1
|
Rationale
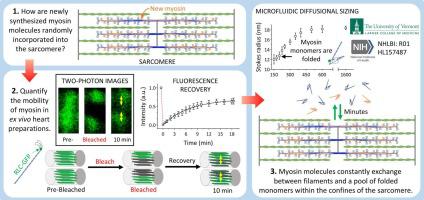
Cardiac muscle cells are terminally differentiated after birth and must beat continually throughout one's lifetime. This mechanical process is driven by the sliding of actin-based thin filaments along myosin-based thick filaments, organized within sarcomeres. Despite costly energetic demand, the half-life of the proteins that comprise the cardiac thick filaments is ∼10 days, with individual molecules being replaced stochastically, by unknown mechanisms.
Objectives
To allow for the stochastic replacement of molecules, we hypothesized that the structure of thick filaments must be highly dynamic in vivo.
Methods and results
To test this hypothesis in adult mouse hearts, we replaced a fraction of the endogenous myosin regulatory light chain (RLC), a component of thick filaments, with GFP-labeled RLC by adeno-associated viral (AAV) transduction. The RLC-GFP was properly localized to the heads of the myosin molecules within thick filaments in ex vivo heart preparations and had no effect on heart size or actin filament siding in vitro. However, the localization of the RLC-GFP molecules was highly mobile, changing its position within the sarcomere on the minute timescale, when quantified by fluorescence recovery after photobleaching (FRAP) using multiphoton microscopy. Interestingly, RLC-GFP mobility was restricted to within the boundaries of single sarcomeres. When cardiomyocytes were lysed, the RLC-GFP remained strongly bound to myosin heavy chain, and the intact myosin molecules adopted a folded, compact configuration, when disassociated from the filaments at physiological ionic conditions.
Conclusions
These data demonstrate that the structure of the thick filament is highly dynamic in the intact heart, with a rate of molecular exchange into and out of thick filaments that is ∼1500 times faster than that required for the replacement of molecules through protein synthesis or degradation.
中文翻译:

离体心脏制剂中心脏粗丝动力学的可视化
基本原理
心肌细胞在出生后就已终末分化,并且必须在人的一生中持续跳动。这一机械过程是由基于肌动蛋白的细丝沿着肌节内组织的基于肌球蛋白的粗丝滑动驱动的。尽管能量需求昂贵,但组成心脏粗丝的蛋白质的半衰期约为 10 天,单个分子通过未知机制被随机替换。
目标
为了允许分子的随机替换,我们假设粗丝的结构在体内必须是高度动态的。
方法和结果
为了在成年小鼠心脏中检验这一假设,我们通过腺相关病毒 (AAV) 转导,用 GFP 标记的 RLC 替换了一部分内源性肌球蛋白调节轻链 (RLC)(粗丝的一个组成部分)。在离体心脏制备物中,RLC-GFP 正确定位于粗丝内肌球蛋白分子的头部,并且对体外心脏大小或肌动蛋白丝壁没有影响。然而,当使用多光子显微镜通过光漂白后荧光恢复(FRAP)进行定量时,RLC-GFP分子的定位具有高度的移动性,在分钟的时间尺度上改变其在肌节内的位置。有趣的是,RLC-GFP 的移动性仅限于单个肌节的边界内。当心肌细胞被裂解时,RLC-GFP 仍然与肌球蛋白重链牢固结合,并且当在生理离子条件下与细丝分离时,完整的肌球蛋白分子采用折叠的紧凑构型。
结论
这些数据表明,粗丝的结构在完整的心脏中是高度动态的,分子进出粗丝的速率比通过蛋白质合成或降解替换分子所需的速率快约 1500 倍。



























 京公网安备 11010802027423号
京公网安备 11010802027423号