Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 3 ) Pub Date : 2023-11-03 , DOI: 10.1016/j.bbagen.2023.130502 Aline Dias da Purificação 1 , Victor Debbas 1 , Leonardo Yuji Tanaka 1 , Gabriele Verônica de Mello Gabriel 1 , João Wosniak Júnior 1 , Tiphany Coralie De Bessa 1 , Sheila Garcia-Rosa 2 , Francisco Rafael Martins Laurindo 1 , Percillia Victoria Santos Oliveira 1
|
Background
The endoplasmic reticulum (ER) transmembrane chaperones DNAJB12(B12) and DNAJB14(B14) are cofactors that cooperate with cytosolic Heat Shock-70 protein (HSC70) facilitating folding/degradation of nascent membrane proteins and supporting the ER-membrane penetration of viral particles. Here, we assessed structural/functional features of B12/B14 with respect to their regulation by ER stress and their involvement in ER stress-mediated protein reflux.
Methods
We investigated the effect of Unfolded Protein Response(UPR)-eliciting drugs on the expression/regulation of B12-B14 and their roles in ER-to-cytosol translocation of Protein Disulfide Isomerase-A1(PDI).
Results
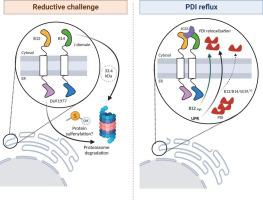
We show that B12 and B14 are similar but do not seem redundant. They share predicted structural features and show high homology of their cytosolic J-domains, while their ER-lumen DUF1977 domains are quite dissimilar. Interactome analysis suggested that B12/B14 associate with different biological processes. UPR activation did not significantly impact on B12 gene expression, while B14 transcripts were up-regulated. Meanwhile, B12 and B14 (33.4 kDa isoform) protein levels were degraded by the proteasome upon acute reductive challenge. Also, B12 degradation was impaired upon sulfenic-acid trapping by dimedone. We originally report that knockdown of B12/B14 and their cytosolic partner SGTA in ER-stressed cells significantly impaired the amount of the ER redox-chaperone PDI in a cytosolic-enriched fraction. Additionally, B12 but not B14 overexpression increased PDI relocalization in non-stressed cells.
Conclusions and general significance
Our findings reveal that B12/B14 regulation involves thiol redox processes that may impact on their stability and possibly on physiological effects. Furthermore, we provide novel evidence that these proteins are involved in UPR-induced ER protein reflux.
中文翻译:

DNAJB12 和 DNJB14 是参与内质网蛋白回流的非冗余 Hsp40 氧化还原伴侣
背景
内质网 (ER) 跨膜伴侣 DNAJB12(B12) 和 DNAJB14(B14) 是与胞质 Heat Shock-70 蛋白 (HSC70) 配合的辅助因子,促进新生膜蛋白的折叠/降解并支持病毒颗粒的 ER 膜穿透。在这里,我们评估了 B12/B14 的结构/功能特征,即它们对 ER 应激的调节以及它们参与 ER 应激介导的蛋白质回流。
方法
我们研究了未折叠蛋白反应 (UPR) 诱导药物对 B12-B14 表达/调节的影响及其在蛋白二硫键异构酶 -A1 (PDI) 内质网到胞质溶胶易位中的作用。
结果
我们证明 B12 和 B14 相似但并不显得多余。它们具有预测的结构特征,并显示出胞质 J 结构域的高度同源性,而它们的 ER 腔 DUF1977 结构域却非常不同。相互作用组分析表明 B12/B14 与不同的生物过程相关。UPR 激活对 B12 基因表达没有显着影响,而 B14 转录本上调。同时,B12 和 B14(33.4 kDa 同工型)蛋白质水平在急性还原攻击后被蛋白酶体降解。此外,双甲酮捕获次磺酸后,B12 的降解也会受到损害。我们最初报道,在内质网应激细胞中敲低 B12/B14 及其胞质伴侣 SGTA 会显着损害胞质富集部分中 ER 氧化还原伴侣 PDI 的量。此外,B12(而非 B14)过表达会增加非应激细胞中的 PDI 重新定位。
结论和一般意义
我们的研究结果表明,B12/B14 调节涉及硫醇氧化还原过程,可能会影响其稳定性并可能影响生理效应。此外,我们提供了新的证据表明这些蛋白质参与 UPR 诱导的 ER 蛋白质反流。



























 京公网安备 11010802027423号
京公网安备 11010802027423号