Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2023-11-11 , DOI: 10.1016/j.yjmcc.2023.11.003 Qianru Jin 1 , Keel Yong Lee 1 , Zoja Selimi 2 , Daisuke Shimura 3 , Ethan Wang 1 , John F Zimmerman 1 , Robin M Shaw 4 , Jan P Kucera 2 , Kevin Kit Parker 1 , Jeffrey E Saffitz 5 , Andre G Kleber 6
|
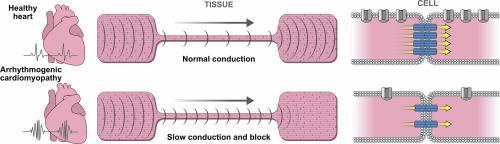
Gap junction and ion channel remodeling occur early in Arrhythmogenic Cardiomyopathy (ACM), but their pathogenic consequences have not been elucidated. Here, we identified the arrhythmogenic substrate, consisting of propagation slowing and conduction block, in ACM models expressing two different desmosomal gene variants. Neonatal rat ventricular myocytes were transduced to express variants in genes encoding desmosomal proteins plakoglobin or plakophilin-2. Studies were performed in engineered cells and anisotropic tissues to quantify changes in conduction velocity, formation of unidirectional propagation, cell–cell electrical coupling, and ion currents. Conduction velocity decreased by 71% and 63% in the two ACM models. SB216763, an inhibitor of glycogen synthase kinase-3 beta, restored conduction velocity to near normal levels. Compared to control, both ACM models showed greater propensity for unidirectional conduction block, which increased further at greater stimulation frequencies. Cell–cell electrical conductance measured in cell pairs was reduced by 86% and 87% in the two ACM models. Computer modeling showed close correspondence between simulated and experimentally determined changes in conduction velocity. The simulation identified that reduced cell–cell electrical coupling was the dominant factor leading to slow conduction, while the combination of reduced cell–cell electrical coupling, reduced sodium current and inward rectifier potassium current explained the development of unidirectional block. Expression of two different ACM variants markedly reduced cell–cell electrical coupling and conduction velocity, and greatly increased the likelihood of developing unidirectional block – both key features of arrhythmogenesis. This study provides the first quantitative analysis of cellular electrophysiological changes leading to the substrate of reentrant arrhythmias in early stage ACM.
中文翻译:

致心律失常性心肌病中电传播和传播阻滞的决定因素
间隙连接和离子通道重塑发生在致心律失常性心肌病(ACM)的早期,但其致病后果尚未阐明。在这里,我们在表达两种不同桥粒基因变体的 ACM 模型中确定了致心律失常的底物,包括传播减慢和传导阻滞。新生大鼠心室肌细胞被转导以表达编码桥粒蛋白plakoglobin或 plakophilin-2 的基因变异体。在工程细胞和各向异性组织中进行了研究,以量化传导速度的变化、单向传播的形成、细胞间电耦合和离子电流。两个 ACM 模型的传导速度分别降低了 71% 和 63%。SB216763 是一种糖原合成酶激酶 3 beta 抑制剂,可将传导速度恢复至接近正常水平。与对照组相比,两种 ACM 模型都表现出更大的单向传导阻滞倾向,并且在刺激频率更高时这种倾向进一步增加。在两个 ACM 模型中,以细胞对测量的细胞间电导率分别降低了 86% 和 87%。计算机建模显示模拟的传导速度变化与实验确定的传导速度变化之间存在密切的对应关系。模拟发现,细胞间电耦合减少是导致传导缓慢的主导因素,而细胞间电耦合减少、钠电流减少和内向整流钾电流的结合解释了单向阻滞的发展。两种不同 ACM 变体的表达显着降低了细胞间的电耦合和传导速度,并大大增加了发生单向阻滞的可能性——这都是心律失常发生的关键特征。这项研究首次对导致早期 ACM 折返性心律失常的细胞电生理变化进行了定量分析。



























 京公网安备 11010802027423号
京公网安备 11010802027423号