当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nonlinear Behavior of Methanol Synthesis Compared to CO2 Methanation
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2023-11-16 , DOI: 10.1002/ceat.202300256 Johannes Leipold 1 , Magnus Jung 2 , Tobias Keßler 1 , Achim Kienle 1, 2
Chemical Engineering & Technology ( IF 2.1 ) Pub Date : 2023-11-16 , DOI: 10.1002/ceat.202300256 Johannes Leipold 1 , Magnus Jung 2 , Tobias Keßler 1 , Achim Kienle 1, 2
Affiliation
|
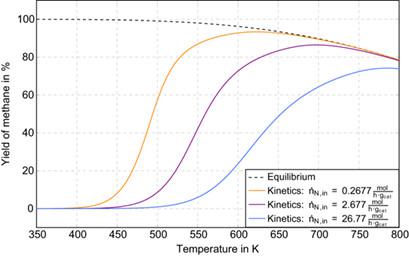
The nonlinear behavior of CO2 methanation over a Ni-catalyst is compared to methanol synthesis over a standard Cu/ZnO-catalyst in a continuous stirred tank reactor (CSTR). Both reactions have received a lot of attention these days for chemical energy storage. Both reactions are exothermic but behave in a different way. CO2 methanation is known to be strongly exothermic, giving rise to multiple steady states. This behavior is induced by the self-acceleration of the methanation reaction because the heat production of the chemical reaction increases with rising temperature. It is shown that methanol synthesis behaves fundamentally different under the operating conditions usually employed in practice. It is less exothermic but, even more important, the overall heat production of the methanol reaction system decreases with increasing temperature, giving rise to a unique and stable steady state. This insight is obtained with an extension of the classical graphical analysis with heat production and heat removal curves.
中文翻译:

甲醇合成与 CO2 甲烷化的非线性行为
将 Ni 催化剂上CO 2甲烷化的非线性行为与连续搅拌釜反应器 (CSTR) 中标准 Cu/ZnO 催化剂上的甲醇合成进行比较。如今,这两种反应在化学储能方面都受到了广泛关注。两种反应都是放热反应,但表现方式不同。已知CO 2甲烷化是强烈放热的,会产生多种稳态。这种行为是由甲烷化反应的自加速引起的,因为化学反应的产热随着温度的升高而增加。结果表明,甲醇合成在实践中通常采用的操作条件下表现出根本不同。它放热较少,但更重要的是,甲醇反应系统的总体产热随着温度的升高而降低,从而产生独特且稳定的稳态。这种见解是通过对产热量和排热量曲线的经典图形分析进行扩展而获得的。
更新日期:2023-11-16
中文翻译:

甲醇合成与 CO2 甲烷化的非线性行为
将 Ni 催化剂上CO 2甲烷化的非线性行为与连续搅拌釜反应器 (CSTR) 中标准 Cu/ZnO 催化剂上的甲醇合成进行比较。如今,这两种反应在化学储能方面都受到了广泛关注。两种反应都是放热反应,但表现方式不同。已知CO 2甲烷化是强烈放热的,会产生多种稳态。这种行为是由甲烷化反应的自加速引起的,因为化学反应的产热随着温度的升高而增加。结果表明,甲醇合成在实践中通常采用的操作条件下表现出根本不同。它放热较少,但更重要的是,甲醇反应系统的总体产热随着温度的升高而降低,从而产生独特且稳定的稳态。这种见解是通过对产热量和排热量曲线的经典图形分析进行扩展而获得的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号