Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2023-11-22 , DOI: 10.1016/j.yjmcc.2023.11.007 Wenke Shi 1 , Jiaojiao Chen 2 , Nan Zhao 1 , Yun Xing 1 , Shiqiang Liu 1 , Mengya Chen 1 , Wenxi Fang 1 , Tong Zhang 1 , Lanlan Li 1 , Heng Zhang 1 , Min Zhang 3 , Xiaofeng Zeng 4 , Si Chen 4 , Shasha Wang 4 , Saiyang Xie 1 , Wei Deng 1
|
Aim
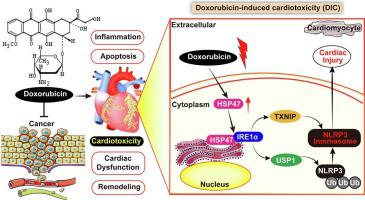
Doxorubicin-induced cardiotoxicity (DIC) is an increasing problem, occurring in many cancer patients receiving anthracycline chemotherapy, ultimately leading to heart failure (HF). Unfortunately, DIC remains difficult to manage due to an ignorance regarding pathophysiological mechanisms. Our work aimed to evaluate the role of HSP47 in doxorubicin-induced HF, and to explore the molecular mechanisms.
Methods and results
Mice were exposed to multi-intraperitoneal injection of doxorubicin (DOX, 4 mg/kg/week, for 6 weeks continuously) to produce DIC. HSP47 expression was significantly upregulated in serum and in heart tissue in DOX-treated mice and in isolated cardiomyocytes. Mice with cardiac-specific HSP47 overexpression and knockdown were generated using recombinant adeno-associated virus (rAVV9) injection. Importantly, cardiac-specific HSP47 overexpression exacerbated cardiac dysfunction in DIC, while HSP47 knockdown prevented DOX-induced cardiac dysfunction, cardiac atrophy and fibrosis in vivo and in vitro. Mechanistically, we identified that HSP47 directly interacted with IRE1α in cardiomyocytes. Furthermore, we provided powerful evidence that HSP47-IRE1α complex promoted TXNIP/NLRP3 inflammasome and reinforced USP1-mediated NLRP3 ubiquitination. Moreover, NLRP3 deficiency in vivo conspicuously abolished HSP47-mediated cardiac atrophy and fibrogenesis under DOX condition.
Conclusion
HSP47 was highly expressed in serum and cardiac tissue after doxorubicin administration. HSP47 contributed to long-term anthracycline chemotherapy-associated cardiac dysfunction in an NLRP3-dependent manner. HSP47 therefore represents a plausible target for future therapy of doxorubicin-induced HF.
中文翻译:

靶向热休克蛋白 47 通过抑制 NLRP3 炎性体减轻阿霉素诱导的小鼠心脏毒性和重塑
目的
阿霉素引起的心脏毒性 (DIC) 是一个日益严重的问题,发生在许多接受蒽环类化疗的癌症患者中,最终导致心力衰竭 (HF)。不幸的是,由于对病理生理机制的无知,DIC 仍然难以治疗。我们的工作旨在评估 HSP47 在阿霉素诱导心力衰竭中的作用,并探讨其分子机制。
方法和结果
小鼠多次腹腔注射阿霉素(DOX,4 mg/kg/周,连续6周)产生DIC。在 DOX 处理的小鼠和分离的心肌细胞中,血清和心脏组织中的 HSP47 表达显着上调。使用重组腺相关病毒 (rAVV9) 注射产生心脏特异性 HSP47 过表达和敲低的小鼠。重要的是,心脏特异性 HSP47 过表达加剧了 DIC 中的心脏功能障碍,而 HSP47 敲除可在体内和体外预防 DOX 诱导的心脏功能障碍、心脏萎缩和纤维化。从机制上讲,我们发现 HSP47 直接与心肌细胞中的 IRE1α 相互作用。此外,我们提供了强有力的证据表明HSP47-IRE1α复合物促进了TXNIP/NLRP3炎症小体并增强了USP1介导的NLRP3泛素化。此外,体内NLRP3缺陷明显消除了DOX条件下HSP47介导的心肌萎缩和纤维化。
结论
阿霉素给药后,HSP47 在血清和心脏组织中高表达。HSP47 以 NLRP3 依赖性方式导致长期蒽环类化疗相关的心功能障碍。因此,HSP47 代表了未来治疗阿霉素诱发心力衰竭的一个合理靶点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号