Journal of Loss Prevention in the Process Industries ( IF 3.5 ) Pub Date : 2023-11-23 , DOI: 10.1016/j.jlp.2023.105225 Shubham K. Das , Vivekanand V. Swami , Ganpati N. Joshi , Prashant S. Kulkarni
|
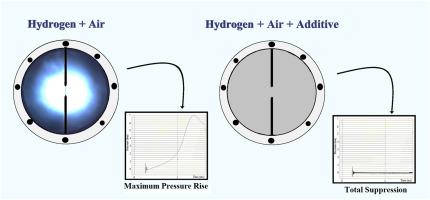
Various approaches are being used for the minimization of risk related to hydrogen hazards. The present study highlights the suppression of hydrogen-air explosion using additives (bromoethane and bromopropane) through experimental and theoretical methods. The experiments were carried out with different equivalence ratios (Ф = 0.5–2) and additive contents (3–15%) at 298 K and 1 bar initial conditions. The results indicate the influence of additives on the maximum pressure-rise, minimum ignition energy and burning velocity. Theoretical study was conducted using CHEMKIN and determined the sensitivity analysis, species profile and reaction path analysis. After the explosion, pressure increases at lower additive fractions for fuel-lean and stoichiometric mixtures and decreases at higher additive contents. In fuel-rich mixtures, pressure rapidly decreases with the increasing additive contents. In the presence of additives, the relative pressure-rise rate decreases, while the time to maximum pressure-rise and ignition energy increase are irrespective of Ф. Moreover, the maximum declination of burning velocity reflects at Φ = 2 and 3% of either additive reduces the burning rate almost by half. The sensitivity coefficient decreases for the flame-promoting reactions and increases for the flame-inhibiting reactions. In such reactions, the inhibiting radicals capture the free radicals and cease the flame propagation, resulting in flame suppression. The reactions R788 (C2H5Br + OH = C2H4Br + H2O) and R834 (C3H7Br + OH = C3H6Br + H2O) have a negative reaction sensitivity coefficient that reflects the effect of suppression. The enhancement of the mole fraction of hydrogen bromide (HBr) with additive content signifies flame suppression. The effect of additives on the explosion characteristics indicates that bromopropane is more effective than bromoethane in minimizing hydrogen hazards.
中文翻译:

使用化学蒸气添加剂抑制氢气-空气混合物
人们正在使用各种方法来尽量减少与氢危害相关的风险。本研究强调通过实验和理论方法使用添加剂(溴乙烷和溴丙烷)抑制氢气-空气爆炸。实验在 298 K 和 1 bar 的初始条件下采用不同的当量比 (Ф = 0.5–2) 和添加剂含量 (3–15%) 进行。结果表明添加剂对最大压升、最小点火能和燃烧速度的影响。使用CHEMKIN进行理论研究并确定敏感性分析、物种概况和反应路径分析。爆炸后,贫燃料和化学计量混合物的添加剂分数较低时压力增加,添加剂含量较高时压力降低。在富含燃料的混合物中,压力随着添加剂含量的增加而迅速降低。在添加剂存在下,相对压力上升速率降低,而达到最大压力上升的时间和点火能量增加与Ф无关。此外,燃烧速度的最大偏角反映在 Φ = 2 时,任何一种添加剂的 3% 都会使燃烧速度几乎降低一半。促进火焰反应的灵敏度系数降低,抑制火焰反应的灵敏度系数增加。在此类反应中,抑制性自由基捕获自由基并停止火焰传播,从而抑制火焰。反应 R788 (C 2 H 5 Br + OH = C 2 H 4 Br + H 2 O) 和 R834 (C 3 H 7 Br + OH = C 3 H 6 Br + H 2 O) 具有负反应灵敏度系数,体现了压制的效果。溴化氢(HBr)摩尔分数随添加剂含量的增加意味着火焰抑制。添加剂对爆炸特性的影响表明,溴丙烷在减少氢危害方面比溴乙烷更有效。



























 京公网安备 11010802027423号
京公网安备 11010802027423号