Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The chemokines CCL5 and CXCL12 exhibit high-affinity binding to N-terminal peptides of the non-cognate receptors CXCR4 and CCR5, respectively
The FEBS Journal ( IF 5.4 ) Pub Date : 2023-11-23 , DOI: 10.1111/febs.17013 Naama Kessler 1 , Sabine R. Akabayov 1 , Leah S. Cohen 2, 3 , Tali Scherf 4 , Fred Naider 1, 2, 3 , Jacob Anglister 1
The FEBS Journal ( IF 5.4 ) Pub Date : 2023-11-23 , DOI: 10.1111/febs.17013 Naama Kessler 1 , Sabine R. Akabayov 1 , Leah S. Cohen 2, 3 , Tali Scherf 4 , Fred Naider 1, 2, 3 , Jacob Anglister 1
Affiliation
|
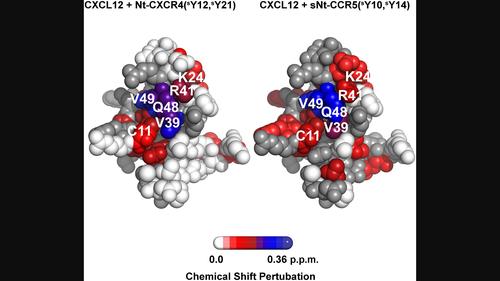
CC and CXC chemokines are distinct chemokine subfamilies. CC chemokines usually do not bind CXC-chemokine receptors and vice versa. CCR5 and CXCR4 receptors are activated by CCL5 and CXCL12 chemokines, respectively, and are also used as HIV-1 coreceptors. CCL5 contains one conserved binding site for a sulfated tyrosine residue, whereas CXCL12 is unique in having two additional sites for sulfated/nonsulfated tyrosine residues. In this study, N-terminal (Nt) CXCR4 peptides were found to bind CCL5 with somewhat higher affinities in comparison to those of short Nt-CCR5(8–20) peptides with the same number of sulfated tyrosine residues. Similarly, a long Nt-CCR5(1–27)(sY3,sY10,sY14) peptide cross reacts with CXCL12 and with lower KD in comparison to its binding to CCL5. Intermolecular nuclear overhauser effect (NOE) measurements were used to decipher the mechanism of the chemokine/Nt-receptor peptide binding. The Nt-CXCR4 peptides interact with the conserved CCL5 tyrosine sulfate-binding site by an allovalency mechanism like that observed for CCL5 binding of Nt-CCR5 peptides. Nt-CCR5 peptides bind CXCL12 in multiple modes analogous to their binding to HIV-1 gp120 and interact with all three tyrosine/sulfated tyrosine-binding pockets of CXCL12. We suggest that the chemokine-receptors Nt-segments bind promiscuously to cognate and non-cognate chemokines and in a mechanism that is dependent on the number of binding pockets for tyrosine residues found on the chemokine. In conclusion, common features shared among the chemokine-receptors' Nt-segments such as multiple tyrosine residues that are potentially sulfated, and a large number of negatively charged residues are the reason of the cross binding observed in this study.
中文翻译:

趋化因子 CCL5 和 CXCL12 分别与非同源受体 CXCR4 和 CCR5 的 N 端肽表现出高亲和力结合
CC 和 CXC 趋化因子是不同的趋化因子亚家族。 CC 趋化因子通常不结合 CXC 趋化因子受体,反之亦然。 CCR5 和 CXCR4 受体分别由 CCL5 和 CXCL12 趋化因子激活,也用作 HIV-1 辅助受体。 CCL5 含有一个硫酸化酪氨酸残基的保守结合位点,而 CXCL12 的独特之处在于具有两个额外的硫酸化/非硫酸化酪氨酸残基位点。在这项研究中,与具有相同数量硫酸化酪氨酸残基的短 Nt-CCR5(8–20) 肽相比,N 端 (Nt) CXCR4 肽与 CCL5 的结合亲和力稍高。同样,长 Nt-CCR5(1–27)( s Y3、s Y10、s Y14) 肽与 CXCL12 发生交叉反应,且与与 CCL5 的结合相比,K D较低。分子间核欧沃豪瑟效应 (NOE) 测量用于破译趋化因子/Nt 受体肽结合的机制。 Nt-CXCR4 肽通过与 Nt-CCR5 肽的 CCL5 结合观察到的同价机制与保守的 CCL5 酪氨酸硫酸盐结合位点相互作用。 Nt-CCR5 肽以多种模式结合 CXCL12,类似于其与 HIV-1 gp120 的结合,并与 CXCL12 的所有三个酪氨酸/硫酸化酪氨酸结合口袋相互作用。我们认为趋化因子受体 Nt 片段与同源和非同源趋化因子混杂结合,其机制取决于趋化因子上发现的酪氨酸残基的结合口袋的数量。总之,趋化因子受体的 Nt 区段之间共有的共同特征,例如可能被硫酸化的多个酪氨酸残基和大量带负电的残基是本研究中观察到的交叉结合的原因。
更新日期:2023-11-23
中文翻译:

趋化因子 CCL5 和 CXCL12 分别与非同源受体 CXCR4 和 CCR5 的 N 端肽表现出高亲和力结合
CC 和 CXC 趋化因子是不同的趋化因子亚家族。 CC 趋化因子通常不结合 CXC 趋化因子受体,反之亦然。 CCR5 和 CXCR4 受体分别由 CCL5 和 CXCL12 趋化因子激活,也用作 HIV-1 辅助受体。 CCL5 含有一个硫酸化酪氨酸残基的保守结合位点,而 CXCL12 的独特之处在于具有两个额外的硫酸化/非硫酸化酪氨酸残基位点。在这项研究中,与具有相同数量硫酸化酪氨酸残基的短 Nt-CCR5(8–20) 肽相比,N 端 (Nt) CXCR4 肽与 CCL5 的结合亲和力稍高。同样,长 Nt-CCR5(1–27)( s Y3、s Y10、s Y14) 肽与 CXCL12 发生交叉反应,且与与 CCL5 的结合相比,K D较低。分子间核欧沃豪瑟效应 (NOE) 测量用于破译趋化因子/Nt 受体肽结合的机制。 Nt-CXCR4 肽通过与 Nt-CCR5 肽的 CCL5 结合观察到的同价机制与保守的 CCL5 酪氨酸硫酸盐结合位点相互作用。 Nt-CCR5 肽以多种模式结合 CXCL12,类似于其与 HIV-1 gp120 的结合,并与 CXCL12 的所有三个酪氨酸/硫酸化酪氨酸结合口袋相互作用。我们认为趋化因子受体 Nt 片段与同源和非同源趋化因子混杂结合,其机制取决于趋化因子上发现的酪氨酸残基的结合口袋的数量。总之,趋化因子受体的 Nt 区段之间共有的共同特征,例如可能被硫酸化的多个酪氨酸残基和大量带负电的残基是本研究中观察到的交叉结合的原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号