Journal of Molecular and Cellular Cardiology ( IF 5 ) Pub Date : 2023-11-24 , DOI: 10.1016/j.yjmcc.2023.11.010 Fiona L Wong 1 , Thomas A Bunch 1 , Victoria C Lepak 1 , Allison L Steedman 1 , Brett A Colson 1
|
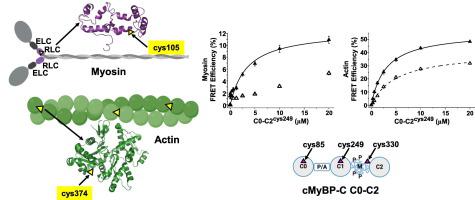
N-terminal cardiac myosin-binding protein C (cMyBP-C) domains (C0-C2) bind to thick (myosin) and thin (actin) filaments to coordinate contraction and relaxation of the heart. These interactions are regulated by phosphorylation of the M-domain situated between domains C1 and C2. In cardiomyopathies and heart failure, phosphorylation of cMyBP-C is significantly altered. We aimed to investigate how cMyBP-C interacts with myosin and actin. We developed complementary, high-throughput, C0-C2 FRET-based binding assays for myosin and actin to characterize the effects due to 5 HCM-linked variants or functional mutations in unphosphorylated and phosphorylated C0-C2. The assays indicated that phosphorylation decreases binding to both myosin and actin, whereas the HCM mutations in M-domain generally increase binding. The effects of mutations were greatest in phosphorylated C0-C2, and some mutations had a larger effect on actin than myosin binding. Phosphorylation also altered the spatial relationship of the probes on C0-C2 and actin. The magnitude of these structural changes was dependent on C0-C2 probe location (C0, C1, or M-domain). We conclude that binding can differ between myosin and actin due to phosphorylation or mutations. Additionally, these variables can change the mode of binding, affecting which of the interactions in cMyBP-C N-terminal domains with myosin or actin take place. The opposite effects of phosphorylation and M-domain mutations is consistent with the idea that cMyBP-C phosphorylation is critical for normal cardiac function. The precision of these assays is indicative of their usefulness in high-throughput screening of drug libraries for targeting cMyBP-C as therapy.
中文翻译:

心肌肌球蛋白结合蛋白 C N 末端与肌球蛋白和肌动蛋白丝的相互作用:磷酸化和 M 结构域突变的相反作用
N 端心肌肌球蛋白结合蛋白 C (cMyBP-C) 结构域 (C0-C2) 与粗(肌球蛋白)和细(肌动蛋白)丝结合,协调心脏的收缩和舒张。这些相互作用受到位于结构域 C1 和 C2 之间的 M 结构域的磷酸化的调节。在心肌病和心力衰竭中,cMyBP-C 的磷酸化显着改变。我们的目的是研究 cMyBP-C 如何与肌球蛋白和肌动蛋白相互作用。我们开发了针对肌球蛋白和肌动蛋白的互补、高通量、基于 C0-C2 FRET 的结合测定,以表征非磷酸化和磷酸化 C0-C2 中 5 个 HCM 相关变异或功能突变造成的影响。测定表明,磷酸化会降低与肌球蛋白和肌动蛋白的结合,而 M 结构域中的 HCM 突变通常会增加结合。突变对磷酸化 C0-C2 的影响最大,一些突变对肌动蛋白的影响比肌球蛋白结合的影响更大。磷酸化还改变了 C0-C2 和肌动蛋白上探针的空间关系。这些结构变化的幅度取决于 C0-C2 探针位置(C0、C1 或 M 结构域)。我们得出的结论是,由于磷酸化或突变,肌球蛋白和肌动蛋白之间的结合可能有所不同。此外,这些变量可以改变结合模式,影响 cMyBP-C N 端结构域与肌球蛋白或肌动蛋白发生的相互作用。磷酸化和 M 结构域突变的相反作用与 cMyBP-C 磷酸化对于正常心脏功能至关重要的观点是一致的。这些测定的精确度表明它们在以 cMyBP-C 为靶向治疗的药物库高通量筛选中的有用性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号