当前位置:
X-MOL 学术
›
Nano-Struct. Nano-Objects
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Properties and performance of Ni(II) doped magnetic Fe3O4@mesoporous SiO2/UiO-66 synthesized by an ultrasound assisted method for potential adsorbent of methyl orange: Kinetic, isotherm and thermodynamic studies
Nano-Structures & Nano-Objects Pub Date : 2023-12-03 , DOI: 10.1016/j.nanoso.2023.101077 Alvin Romadhoni Putra Hidayat , Liyana Labiba Zulfa , Nuhaa Faaizatunnisa , Didik Prasetyoko , Djoko Hartanto , Nurul Widiastuti , Adi Setyo Purnomo , Miftahul Jannah , Etty Nurlia Kusumawati , Hasliza Bahruji , Ratna Ediati
Nano-Structures & Nano-Objects Pub Date : 2023-12-03 , DOI: 10.1016/j.nanoso.2023.101077 Alvin Romadhoni Putra Hidayat , Liyana Labiba Zulfa , Nuhaa Faaizatunnisa , Didik Prasetyoko , Djoko Hartanto , Nurul Widiastuti , Adi Setyo Purnomo , Miftahul Jannah , Etty Nurlia Kusumawati , Hasliza Bahruji , Ratna Ediati
|
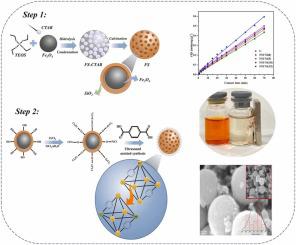
Ni(II) doped magnetic FeO@mesoporous SiO/UiO-66 composite (FSUNi(x)) was synthesized by ultrasound methods for methyl orange (MO) adsorption. Ultrasound irradiation caused the in situ growth of Ni on FeO @mesoporous SiO/UiO-66 composite. Ni enhanced the surface area and porosity of composites due to the substitution of Zr in UiO-66 with Ni, which generates defects. The SEM and TEM analysis exhibited the FeO @mesoporous SiO core of ∼324 nm diameter, coated with a Ni-UiO-66 shell of ∼ 23 nm thickness. At 10% nickel doping, FSUNi(10) composites showed high surface area, large pore diameter, excellent adsorption capacity, and high magnetic properties (8.16 emu/g) that achieved rapid recovery in 20 s. The influence of contact time (1–120 min), dye concentration (25–350 mg/L), pH of the initial solution (2−13), adsorption temperature (30–50 °C), and ionic strength (0–3 g/L) on the adsorption capacity were determined to understand the kinetic and thermodynamic of adsorption. The maximum adsorption capacity increased from 200.803 to 256.410 mg/g with increasing temperature from 30° to 50°C, and adsorption reached optimum capacity at pH 4. According to isothermal and kinetic analyses, adsorption follows the Langmuir isotherm model and the pseudo second order kinetic model. Based on the Langmuir isotherm model, the maximum adsorption capacities of UiO-66 and FSUNi(10) were determined at 182.815 and 200.803 mg/g, respectively. Thermodynamic analysis revealed the spontaneous and endothermic adsorption of MO dye on FSUNi(10). FSUNi(10) showed high adsorption capacity after three regeneration cycles, which can be a potential magnetic adsorbent for wastewater treatment.
中文翻译:

超声波辅助法合成的 Ni(II) 掺杂磁性 Fe3O4@介孔 SiO2/UiO-66 的性质和性能,用于潜在的甲基橙吸附剂:动力学、等温线和热力学研究
采用超声法合成了 Ni(II) 掺杂磁性 FeO@介孔 SiO/UiO-66 复合材料 (FSUNi(x)),用于甲基橙 (MO) 吸附。超声辐照导致 Ni 在 Fe3O@介孔 SiO/UiO-66 复合材料上原位生长。由于 UiO-66 中的 Zr 被 Ni 取代,Ni 增加了复合材料的表面积和孔隙率,从而产生缺陷。SEM 和 TEM 分析显示 Fe3O@介孔 SiO 核直径约为 324 nm,涂有厚度约为 23 nm 的 Ni-UiO-66 壳。当镍掺杂量为10%时,FSUNi(10)复合材料表现出高比表面积、大孔径、优异的吸附能力和高磁性能(8.16 emu/g),可在20 s内实现快速恢复。接触时间(1-120 分钟)、染料浓度(25-350 mg/L)、初始溶液 pH(2-13)、吸附温度(30-50 °C)和离子强度(0- 3 g/L)对吸附容量的影响进行了测定,以了解吸附的动力学和热力学。随着温度从30°C升高到50°C,最大吸附容量从200.803 mg/g增加到256.410 mg/g,并且在pH 4时吸附达到最佳容量。根据等温线和动力学分析,吸附遵循Langmuir等温线模型和伪二级方程。动力学模型。根据Langmuir等温线模型,确定UiO-66和FSUNi(10)的最大吸附容量分别为182.815和200.803 mg/g。热力学分析揭示了MO染料在FSUNi(10)上的自发吸附和吸热吸附。FSUNi(10)经过3次再生循环后表现出较高的吸附能力,可作为一种潜在的废水处理磁性吸附剂。
更新日期:2023-12-03
中文翻译:

超声波辅助法合成的 Ni(II) 掺杂磁性 Fe3O4@介孔 SiO2/UiO-66 的性质和性能,用于潜在的甲基橙吸附剂:动力学、等温线和热力学研究
采用超声法合成了 Ni(II) 掺杂磁性 FeO@介孔 SiO/UiO-66 复合材料 (FSUNi(x)),用于甲基橙 (MO) 吸附。超声辐照导致 Ni 在 Fe3O@介孔 SiO/UiO-66 复合材料上原位生长。由于 UiO-66 中的 Zr 被 Ni 取代,Ni 增加了复合材料的表面积和孔隙率,从而产生缺陷。SEM 和 TEM 分析显示 Fe3O@介孔 SiO 核直径约为 324 nm,涂有厚度约为 23 nm 的 Ni-UiO-66 壳。当镍掺杂量为10%时,FSUNi(10)复合材料表现出高比表面积、大孔径、优异的吸附能力和高磁性能(8.16 emu/g),可在20 s内实现快速恢复。接触时间(1-120 分钟)、染料浓度(25-350 mg/L)、初始溶液 pH(2-13)、吸附温度(30-50 °C)和离子强度(0- 3 g/L)对吸附容量的影响进行了测定,以了解吸附的动力学和热力学。随着温度从30°C升高到50°C,最大吸附容量从200.803 mg/g增加到256.410 mg/g,并且在pH 4时吸附达到最佳容量。根据等温线和动力学分析,吸附遵循Langmuir等温线模型和伪二级方程。动力学模型。根据Langmuir等温线模型,确定UiO-66和FSUNi(10)的最大吸附容量分别为182.815和200.803 mg/g。热力学分析揭示了MO染料在FSUNi(10)上的自发吸附和吸热吸附。FSUNi(10)经过3次再生循环后表现出较高的吸附能力,可作为一种潜在的废水处理磁性吸附剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号