当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
bis-Silyl-triazenide ligands in alkaline-earth metal chemistry
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2023-12-05 , DOI: 10.1002/zaac.202300226 Christian Knüpfer 1 , Jens Langer 1 , Sjoerd Harder 2
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2023-12-05 , DOI: 10.1002/zaac.202300226 Christian Knüpfer 1 , Jens Langer 1 , Sjoerd Harder 2
Affiliation
|
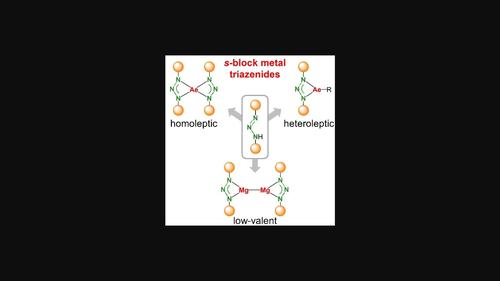
Monoanionic N,N-chelating triazenide ligands, [R-NNN-R]−, are compared to formamidinate or β-diketiminate ligands considerably less electron-donating and therefore potentially suitable for stabilizing electron rich metals in low oxidations states. A feature reported for silyl-substituted triazenide ligands, is their ability to eliminate N2 resulting in (R3Si)2N− anions. Here we describe a series of group 1 and 2 metal complexes with the very bulky bis-silyl-triazenide ligand [tBu3Si-NNN-SitBu3]−. Heteroleptic complexes could be isolated for Mg: {[(tBu3Si)2N3]MgnBu}2 or {[(tBu3Si)2N3]MgI}2, including its ether adducts. Despite bulky silyl substituents, the ligand did not stabilize heteroleptic [(tBu3Si)2N3]AeN(SiMe3)2 complexes for Ca, Sr and Ba and only homoleptic Ae[(tBu3Si)2N3]2 complexes were isolated. Thermal decomposition of these complexes did result in N2 elimination and formation of the expected amide complexes. Reduction of [(tBu3Si)2N3]MgI ⋅ (Et2O) with KC8 in Et2O led to the formation of a MgI complex with a Mg−Mg bond length of 2.902(1) Å. As only one of the Mg centres shows coordination with a Et2O ligand, this is a rare example of an asymmetric Mg−Mg bond. This MgI complex is rather unstable in benzene solution, likely due to reduction of the ligand system. Triazenide ligands are therefore not suitable for stabilization of group 2 metals in low oxidation states.
中文翻译:

碱土金属化学中的双甲硅烷基三氮烯化物配体
与甲脒或β-二酮亚胺配体相比,单阴离子N,N-螯合三氮烯化物配体[R-NNN-R] -的电子供给要少得多,因此可能适合在低氧化态下稳定富电子金属。据报道,甲硅烷基取代的三氮烯化物配体的一个特征是它们能够消除N 2,从而产生(R 3 Si) 2 N -阴离子。在这里,我们描述了一系列具有非常大的双甲硅烷基三氮烯配体 [ t Bu 3 Si-NNN-Si t Bu 3 ] -的第 1 族和第 2 族金属配合物。可以分离出镁的杂配配合物:{[( t Bu 3 Si) 2 N 3 ]Mg n Bu} 2或{[( t Bu 3 Si) 2 N 3 ]MgI} 2,包括其醚加合物。尽管存在大量的甲硅烷基取代基,该配体并不能稳定Ca、Sr和Ba的杂配[( t Bu 3 Si) 2 N 3 ]AeN(SiMe 3 ) 2配合物,并且只能稳定均配Ae[( t Bu 3 Si) 2 N 3 ]分离出2个复合物。这些络合物的热分解确实导致N 2消除并形成预期的酰胺络合物。在Et 2 O 中用 KC 8还原 [( t Bu 3 Si) 2 N 3 ]MgI ⋅ (Et 2 O)导致形成 Mg I络合物,其 Mg−Mg 键长为 2.902(1) Å 。由于只有一个 Mg 中心显示出与 Et 2 O 配体的配位,因此这是不对称 Mg−Mg 键的罕见例子。该 Mg I络合物在苯溶液中相当不稳定,可能是由于配体系统的还原。因此,三氮烯化物配体不适合稳定低氧化态的第2族金属。
更新日期:2023-12-05
中文翻译:

碱土金属化学中的双甲硅烷基三氮烯化物配体
与甲脒或β-二酮亚胺配体相比,单阴离子N,N-螯合三氮烯化物配体[R-NNN-R] -的电子供给要少得多,因此可能适合在低氧化态下稳定富电子金属。据报道,甲硅烷基取代的三氮烯化物配体的一个特征是它们能够消除N 2,从而产生(R 3 Si) 2 N -阴离子。在这里,我们描述了一系列具有非常大的双甲硅烷基三氮烯配体 [ t Bu 3 Si-NNN-Si t Bu 3 ] -的第 1 族和第 2 族金属配合物。可以分离出镁的杂配配合物:{[( t Bu 3 Si) 2 N 3 ]Mg n Bu} 2或{[( t Bu 3 Si) 2 N 3 ]MgI} 2,包括其醚加合物。尽管存在大量的甲硅烷基取代基,该配体并不能稳定Ca、Sr和Ba的杂配[( t Bu 3 Si) 2 N 3 ]AeN(SiMe 3 ) 2配合物,并且只能稳定均配Ae[( t Bu 3 Si) 2 N 3 ]分离出2个复合物。这些络合物的热分解确实导致N 2消除并形成预期的酰胺络合物。在Et 2 O 中用 KC 8还原 [( t Bu 3 Si) 2 N 3 ]MgI ⋅ (Et 2 O)导致形成 Mg I络合物,其 Mg−Mg 键长为 2.902(1) Å 。由于只有一个 Mg 中心显示出与 Et 2 O 配体的配位,因此这是不对称 Mg−Mg 键的罕见例子。该 Mg I络合物在苯溶液中相当不稳定,可能是由于配体系统的还原。因此,三氮烯化物配体不适合稳定低氧化态的第2族金属。



























 京公网安备 11010802027423号
京公网安备 11010802027423号