Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The crystal structure of RsSymEG1 reveals a unique form of smaller GH7 endoglucanases alongside GH7 cellobiohydrolases in protist symbionts of termites
The FEBS Journal ( IF 5.4 ) Pub Date : 2023-12-10 , DOI: 10.1111/febs.17029 Topi Haataja 1 , Henrik Hansson 1 , Shigeharu Moriya 2 , Mats Sandgren 1 , Jerry Ståhlberg 1
The FEBS Journal ( IF 5.4 ) Pub Date : 2023-12-10 , DOI: 10.1111/febs.17029 Topi Haataja 1 , Henrik Hansson 1 , Shigeharu Moriya 2 , Mats Sandgren 1 , Jerry Ståhlberg 1
Affiliation
|
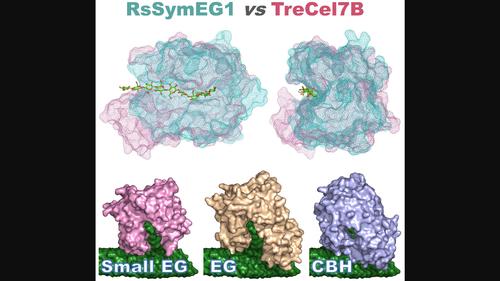
Glycoside hydrolase family 7 (GH7) cellulases are key enzymes responsible for carbon cycling on earth through their role in cellulose degradation and constitute highly important industrial enzymes as well. Although these enzymes are found in a wide variety of evolutionarily distant organisms across eukaryotes, they exhibit remarkably conserved features within two groups: exo-acting cellobiohydrolases and endoglucanases. However, recently reports have emerged of a separate clade of GH7 endoglucanases from protist symbionts of termites that are 60–80 amino acids shorter. In this work, we describe the first crystal structure of a short GH7 endoglucanase, RsSymEG1, from a symbiont of the lower termite Reticulitermes speratus. A more open flat surface and shorter loops around the non-reducing end of the cellulose-binding cleft indicate enhanced access to cellulose chains on the surface of cellulose microfibrils. Additionally, when comparing activities on polysaccharides to a typical fungal GH7 endoglucanase (Trichoderma longibrachiatum Cel7B), RsSymEG1 showed significantly faster initial hydrolytic activity. We also examine the prevalence and diversity of GH7 enzymes that the symbionts provide to the termite host, compare overall structures and substrate binding between cellobiohydrolase and long and short endoglucanase, and highlight the presence of similar short GH7s in other organisms.
中文翻译:

RsSymEG1的晶体结构揭示了白蚁原生共生体中较小的GH7内切葡聚糖酶与GH7纤维二糖水解酶的独特形式
糖苷水解酶家族 7 (GH7) 纤维素酶是通过其在纤维素降解中的作用而负责地球碳循环的关键酶,并且也是非常重要的工业酶。尽管这些酶存在于真核生物中多种进化距离较远的生物体中,但它们在两类中表现出非常保守的特征:外切纤维二糖水解酶和内切葡聚糖酶。然而,最近有报道称,白蚁原生共生体中存在一个单独的 GH7 内切葡聚糖酶进化枝,其长度缩短了 60-80 个氨基酸。在这项工作中,我们描述了来自低等白蚁散白蚁共生体的短 GH7 内切葡聚糖酶 RsSymEG1 的第一个晶体结构。更开放的平坦表面和围绕纤维素结合裂口的非还原端的更短的环表明纤维素微纤维表面上的纤维素链的接触增强。此外,当将多糖活性与典型真菌 GH7 内切葡聚糖酶(长木霉Cel7B)进行比较时,RsSymEG1 显示出明显更快的初始水解活性。我们还检查了共生体向白蚁宿主提供的 GH7 酶的普遍性和多样性,比较了纤维二糖水解酶与长和短内切葡聚糖酶之间的整体结构和底物结合,并强调了其他生物体中类似的短 GH7 的存在。
更新日期:2023-12-10
中文翻译:

RsSymEG1的晶体结构揭示了白蚁原生共生体中较小的GH7内切葡聚糖酶与GH7纤维二糖水解酶的独特形式
糖苷水解酶家族 7 (GH7) 纤维素酶是通过其在纤维素降解中的作用而负责地球碳循环的关键酶,并且也是非常重要的工业酶。尽管这些酶存在于真核生物中多种进化距离较远的生物体中,但它们在两类中表现出非常保守的特征:外切纤维二糖水解酶和内切葡聚糖酶。然而,最近有报道称,白蚁原生共生体中存在一个单独的 GH7 内切葡聚糖酶进化枝,其长度缩短了 60-80 个氨基酸。在这项工作中,我们描述了来自低等白蚁散白蚁共生体的短 GH7 内切葡聚糖酶 RsSymEG1 的第一个晶体结构。更开放的平坦表面和围绕纤维素结合裂口的非还原端的更短的环表明纤维素微纤维表面上的纤维素链的接触增强。此外,当将多糖活性与典型真菌 GH7 内切葡聚糖酶(长木霉Cel7B)进行比较时,RsSymEG1 显示出明显更快的初始水解活性。我们还检查了共生体向白蚁宿主提供的 GH7 酶的普遍性和多样性,比较了纤维二糖水解酶与长和短内切葡聚糖酶之间的整体结构和底物结合,并强调了其他生物体中类似的短 GH7 的存在。



























 京公网安备 11010802027423号
京公网安备 11010802027423号