当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Preclinical and dose-ranging assessment of hESC-derived dopaminergic progenitors for a clinical trial on Parkinson's disease
Cell Stem Cell ( IF 23.9 ) Pub Date : 2023-12-11 , DOI: 10.1016/j.stem.2023.11.009 Sanghyun Park , Chan Wook Park , Jang Hyeon Eom , Mi-Young Jo , Hye-Jin Hur , Sung Kyoung Choi , Jae Souk Lee , Seung Taek Nam , Ki-Sang Jo , Young Woo Oh , Jungil Lee , Sieun Kim , Do-Hun Kim , Chul-Yong Park , Su Jin Kim , Ho-Young Lee , Myung Soo Cho , Dae-Sung Kim , Dong-Wook Kim
Cell Stem Cell ( IF 23.9 ) Pub Date : 2023-12-11 , DOI: 10.1016/j.stem.2023.11.009 Sanghyun Park , Chan Wook Park , Jang Hyeon Eom , Mi-Young Jo , Hye-Jin Hur , Sung Kyoung Choi , Jae Souk Lee , Seung Taek Nam , Ki-Sang Jo , Young Woo Oh , Jungil Lee , Sieun Kim , Do-Hun Kim , Chul-Yong Park , Su Jin Kim , Ho-Young Lee , Myung Soo Cho , Dae-Sung Kim , Dong-Wook Kim
|
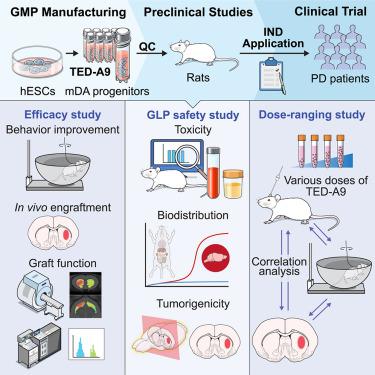
Human embryonic stem cell (hESC)-derived midbrain dopaminergic (mDA) cell transplantation is a promising therapeutic strategy for Parkinson’s disease (PD). Here, we present the derivation of high-purity mDA progenitors from clinical-grade hESCs on a large scale under rigorous good manufacturing practice (GMP) conditions. We also assessed the toxicity, biodistribution, and tumorigenicity of these cells in immunodeficient rats in good laboratory practice (GLP)-compliant facilities. Various doses of mDA progenitors were transplanted into hemi-parkinsonian rats, and a significant dose-dependent behavioral improvement was observed with a minimal effective dose range of 5,000–10,000 mDA progenitor cells. These results provided insights into determining a low cell dosage (3.15 million cells) for human clinical trials. Based on these results, approval for a phase 1/2a clinical trial for PD cell therapy was obtained from the Ministry of Food and Drug Safety in Korea, and a clinical trial for treating patients with PD has commenced.
中文翻译:

用于帕金森病临床试验的 hESC 衍生多巴胺能祖细胞的临床前和剂量范围评估
人胚胎干细胞(hESC)来源的中脑多巴胺能(mDA)细胞移植是治疗帕金森病(PD)的一种有前途的治疗策略。在这里,我们展示了在严格的良好生产规范 (GMP) 条件下从临床级 hESC 大规模衍生高纯度 mDA 祖细胞的方法。我们还在符合良好实验室规范 (GLP) 的设施中评估了这些细胞在免疫缺陷大鼠中的毒性、生物分布和致瘤性。将不同剂量的 mDA 祖细胞移植到偏侧帕金森病大鼠体内,在 5,000-10,000 个 mDA 祖细胞的最小有效剂量范围内观察到显着的剂量依赖性行为改善。这些结果为确定人体临床试验的低细胞剂量(315 万个细胞)提供了见解。基于这些结果,PD细胞疗法的1/2a期临床试验获得韩国食品药品安全部的批准,治疗PD患者的临床试验已经开始。
更新日期:2023-12-11
中文翻译:

用于帕金森病临床试验的 hESC 衍生多巴胺能祖细胞的临床前和剂量范围评估
人胚胎干细胞(hESC)来源的中脑多巴胺能(mDA)细胞移植是治疗帕金森病(PD)的一种有前途的治疗策略。在这里,我们展示了在严格的良好生产规范 (GMP) 条件下从临床级 hESC 大规模衍生高纯度 mDA 祖细胞的方法。我们还在符合良好实验室规范 (GLP) 的设施中评估了这些细胞在免疫缺陷大鼠中的毒性、生物分布和致瘤性。将不同剂量的 mDA 祖细胞移植到偏侧帕金森病大鼠体内,在 5,000-10,000 个 mDA 祖细胞的最小有效剂量范围内观察到显着的剂量依赖性行为改善。这些结果为确定人体临床试验的低细胞剂量(315 万个细胞)提供了见解。基于这些结果,PD细胞疗法的1/2a期临床试验获得韩国食品药品安全部的批准,治疗PD患者的临床试验已经开始。



























 京公网安备 11010802027423号
京公网安备 11010802027423号