当前位置:
X-MOL 学术
›
Adv. Therap.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering Living Immunotherapeutic Agents for Improved Cancer Treatment
Advanced Therapeutics ( IF 4.6 ) Pub Date : 2023-12-18 , DOI: 10.1002/adtp.202300302 T. Gwisai 1 , S. Günther 1 , N. Mirkhani 1 , M. Vizovisek 1 , S. Menghini 1 , M. Jacobs 1 , M. G. Christiansen 1 , I. Oberhuber 1 , P. Poc 1 , S. Schuerle 1
Advanced Therapeutics ( IF 4.6 ) Pub Date : 2023-12-18 , DOI: 10.1002/adtp.202300302 T. Gwisai 1 , S. Günther 1 , N. Mirkhani 1 , M. Vizovisek 1 , S. Menghini 1 , M. Jacobs 1 , M. G. Christiansen 1 , I. Oberhuber 1 , P. Poc 1 , S. Schuerle 1
Affiliation
|
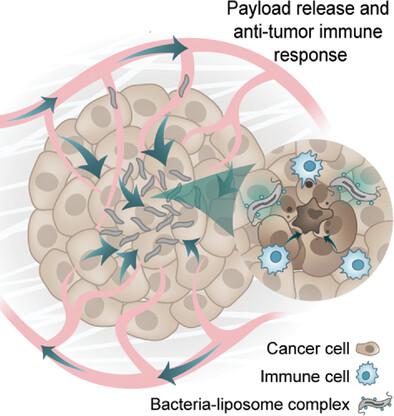
Bacteria-based agents are emerging as promising tools for cancer therapy due to their ability to actively target tumors, trigger localized inflammation, and induce tumor regression. There has been growing interest in using bacteria that are responsive to external cues, such as magnetic fields, to facilitate the formation of robust colonies in tumors and achieve the threshold for clinical efficacy. Several studies have demonstrated the potential of innately magnetically responsive bacteria, known as magnetotactic bacteria (MTB), as steerable agents. However, their immunostimulatory properties, which play a central role in their function as therapeutic agents, have not yet been adequately studied. Here, key aspects of human immune cell response to MTB strain Magnetospirillum magneticum AMB-1 in physiological environments are characterized. The ability of MTB to maintain magnetic properties, remain viable in whole blood, elicit cytokine production by macrophages, and stimulate uptake of cancer cell material by dendritic cells is examined. This study also investigates the use of MTB–liposome complexes for effective delivery of therapeutic payloads in vitro and explores response to the agent in vivo. Overall, this work establishes the potential of MTB as a versatile, combined delivery platform for immune-mediated cancer therapy.
中文翻译:

设计活体免疫治疗剂以改善癌症治疗
基于细菌的药物因其能够主动靶向肿瘤、引发局部炎症和诱导肿瘤消退而成为癌症治疗的有前景的工具。人们越来越感兴趣使用对外部信号(例如磁场)做出反应的细菌来促进肿瘤中强大的菌落的形成并达到临床疗效的阈值。几项研究证明了固有磁响应细菌(称为趋磁细菌(MTB))作为可操纵剂的潜力。然而,它们的免疫刺激特性在其作为治疗剂的功能中发挥着核心作用,但尚未得到充分研究。在此,描述了生理环境中人体免疫细胞对 MTB 菌株MagnetospirillumMagneum AMB-1 反应的关键方面。检查了 MTB 保持磁性、在全血中保持活力、引发巨噬细胞产生细胞因子以及刺激树突状细胞摄取癌细胞材料的能力。这项研究还研究了 MTB-脂质体复合物在体外有效递送治疗有效负载的用途,并探讨了该药物在体内的反应。总体而言,这项工作确立了 MTB 作为免疫介导癌症治疗的多功能组合递送平台的潜力。
更新日期:2023-12-18
中文翻译:

设计活体免疫治疗剂以改善癌症治疗
基于细菌的药物因其能够主动靶向肿瘤、引发局部炎症和诱导肿瘤消退而成为癌症治疗的有前景的工具。人们越来越感兴趣使用对外部信号(例如磁场)做出反应的细菌来促进肿瘤中强大的菌落的形成并达到临床疗效的阈值。几项研究证明了固有磁响应细菌(称为趋磁细菌(MTB))作为可操纵剂的潜力。然而,它们的免疫刺激特性在其作为治疗剂的功能中发挥着核心作用,但尚未得到充分研究。在此,描述了生理环境中人体免疫细胞对 MTB 菌株MagnetospirillumMagneum AMB-1 反应的关键方面。检查了 MTB 保持磁性、在全血中保持活力、引发巨噬细胞产生细胞因子以及刺激树突状细胞摄取癌细胞材料的能力。这项研究还研究了 MTB-脂质体复合物在体外有效递送治疗有效负载的用途,并探讨了该药物在体内的反应。总体而言,这项工作确立了 MTB 作为免疫介导癌症治疗的多功能组合递送平台的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号