当前位置:
X-MOL 学术
›
J. Autoimmun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gene-expression profiling of laser-dissected islets and studies in deficient mice reveal chemokines as differential driving force of type 1 diabetes
Journal of Autoimmunity ( IF 12.8 ) Pub Date : 2023-12-22 , DOI: 10.1016/j.jaut.2023.103161 Christine Bender , Peter Müller , Camilla Tondello , Jessica Horn , Martin Holdener , Stanley Lasch , Monika Bayer , Josef M. Pfeilschifter , Frank Tacke , Andreas Ludwig , Martin-Leo Hansmann , Claudia Döring , Edith Hintermann , Urs Christen
Journal of Autoimmunity ( IF 12.8 ) Pub Date : 2023-12-22 , DOI: 10.1016/j.jaut.2023.103161 Christine Bender , Peter Müller , Camilla Tondello , Jessica Horn , Martin Holdener , Stanley Lasch , Monika Bayer , Josef M. Pfeilschifter , Frank Tacke , Andreas Ludwig , Martin-Leo Hansmann , Claudia Döring , Edith Hintermann , Urs Christen
|
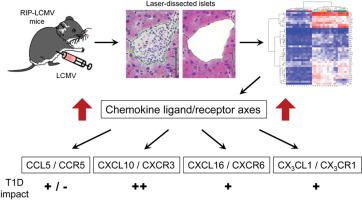
Although type 1 diabetes (T1D) results from the autoimmune destruction of the insulin-producing β-cells, its treatment is largely restricted to exogenous insulin administration. Only few therapies targeting the autoaggressive immune system have been introduced into clinical practice or are considered in clinical trials. Here, we provide a gene expression profile of the islet microenvironment obtained by laser-dissection microscopy in an inducible mouse model. Thereby, we have identified novel targets for immune intervention. Increased gene expression of most inflammatory proteins was apparent at day 10 after T1D induction and largely paralleled the observed degree of insulitis. We further focused on genes involved in leukocyte migration, including chemokines and their receptors. Besides the critical chemokine CXCL10, we found several other chemokines upregulated locally in temporary or chronic manner. Localization of the chemokine ligand/receptor pairs to the islet microenvironment has been confirmed by RNAscope. Interference with the CXCL16-CXCR6 and CXCL1-CXCR1 axes, but not the CCL5-CCR1/3/5 axis, resulted in reduced insulitis and lower T1D incidence. Further, we found that the receptors for the differentially expressed chemokines CXCL10, CXCL16 and CXCL1 are distributed unevenly among islet autoantigen-specific T cells, which explains why the interference with just one chemokine axis cannot completely abrogate insulitis and T1D.
中文翻译:

激光解剖胰岛的基因表达谱和缺陷小鼠的研究揭示趋化因子是 1 型糖尿病的差异驱动力
尽管 1 型糖尿病 (T1D) 是由产生胰岛素的 β 细胞的自身免疫性破坏引起的,但其治疗很大程度上仅限于外源性胰岛素给药。只有少数针对自身攻击性免疫系统的疗法已被引入临床实践或在临床试验中考虑。在这里,我们提供了通过激光解剖显微镜在诱导小鼠模型中获得的胰岛微环境的基因表达谱。因此,我们确定了免疫干预的新靶点。大多数炎症蛋白的基因表达增加在 T1D 诱导后第 10 天很明显,并且与观察到的胰岛炎程度基本一致。我们进一步关注参与白细胞迁移的基因,包括趋化因子及其受体。除了关键的趋化因子 CXCL10 之外,我们还发现其他几种趋化因子以暂时或慢性的方式局部上调。 RNAscope 已证实趋化因子配体/受体对在胰岛微环境中的定位。干扰 CXCL16-CXCR6 和 CXCL1-CXCR1 轴(而非 CCL5-CCR1/3/5 轴)可减少胰岛炎并降低 T1D 发病率。此外,我们发现差异表达的趋化因子CXCL10、CXCL16和CXCL1的受体在胰岛自身抗原特异性T细胞中分布不均匀,这解释了为什么仅干扰一种趋化因子轴不能完全消除胰岛炎和T1D。
更新日期:2023-12-22
中文翻译:

激光解剖胰岛的基因表达谱和缺陷小鼠的研究揭示趋化因子是 1 型糖尿病的差异驱动力
尽管 1 型糖尿病 (T1D) 是由产生胰岛素的 β 细胞的自身免疫性破坏引起的,但其治疗很大程度上仅限于外源性胰岛素给药。只有少数针对自身攻击性免疫系统的疗法已被引入临床实践或在临床试验中考虑。在这里,我们提供了通过激光解剖显微镜在诱导小鼠模型中获得的胰岛微环境的基因表达谱。因此,我们确定了免疫干预的新靶点。大多数炎症蛋白的基因表达增加在 T1D 诱导后第 10 天很明显,并且与观察到的胰岛炎程度基本一致。我们进一步关注参与白细胞迁移的基因,包括趋化因子及其受体。除了关键的趋化因子 CXCL10 之外,我们还发现其他几种趋化因子以暂时或慢性的方式局部上调。 RNAscope 已证实趋化因子配体/受体对在胰岛微环境中的定位。干扰 CXCL16-CXCR6 和 CXCL1-CXCR1 轴(而非 CCL5-CCR1/3/5 轴)可减少胰岛炎并降低 T1D 发病率。此外,我们发现差异表达的趋化因子CXCL10、CXCL16和CXCL1的受体在胰岛自身抗原特异性T细胞中分布不均匀,这解释了为什么仅干扰一种趋化因子轴不能完全消除胰岛炎和T1D。



























 京公网安备 11010802027423号
京公网安备 11010802027423号