当前位置:
X-MOL 学术
›
FEBS Journal
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization and structural study of a novel β-N-acetylgalactosaminidase from Niabella aurantiaca
FEBS Journal Pub Date : 2023-12-21 , DOI: 10.1111/febs.17042 Eduardo S. Moreno Prieto 1 , Sune Fjermedal 1 , Suzana Siebenhaar 1 , Marlène Vuillemin 1 , Jesper Holck 1 , Renaud Vincentelli 2 , Garry P. Gippert 1 , Casper Wilkens 1 , Jens Preben Morth 1 , Bernard Henrissat 1, 3
FEBS Journal Pub Date : 2023-12-21 , DOI: 10.1111/febs.17042 Eduardo S. Moreno Prieto 1 , Sune Fjermedal 1 , Suzana Siebenhaar 1 , Marlène Vuillemin 1 , Jesper Holck 1 , Renaud Vincentelli 2 , Garry P. Gippert 1 , Casper Wilkens 1 , Jens Preben Morth 1 , Bernard Henrissat 1, 3
Affiliation
|
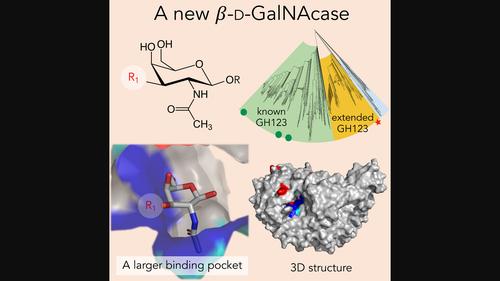
We report here the identification, characterization and three-dimensional (3D) structure determination of NaNga, a newly identified β-N-acetylgalactosaminidase from the Gram-negative soil bacterium Niabella aurantiaca DSM 17617. When recombinantly expressed in Escherichia coli, the enzyme selectively cleaved 4-nitrophenyl-N-acetyl-β-d-galactosamine (pNP-β-d-GalpNAc). The X-ray crystal structure of the protein was refined to 2.5 Å and consists of an N-terminal β-sandwich domain and a (β/α)8 barrel catalytic domain. Despite a mere 22% sequence identity, the 3D structure of NaNga is similar to those previously determined for family GH123 members, suggesting it also employs the same substrate-assisted catalytic mechanism. Inhibition by N-acetyl-galactosamine thiazoline (GalNAc-thiazoline) supports the suggested mechanism. A phylogenetic analysis of its proximal sequence space shows significant clustering of unknown sequences around NaNga with sufficient divergence with previously identified GH123 members to subdivide this family into distinct subfamilies. Although the actual biological substrate of our enzyme remains unknown, examination of the active site pocket suggests that it may be a β-N-acetylgalactosaminide substituted by a monosaccharide at O-3. Analysis of the genomic context suggests, in turn, that this substituted β-N-acetylgalactosaminide may be appended to a d-arabinan from an environmental Actinomycete.
中文翻译:

一种来自 Niabella aurantiaca 的新型 β-N-乙酰半乳糖胺酶的表征和结构研究
我们在此报告了Na Nga的鉴定、表征和三维 (3D) 结构测定,Na Nga 是一种新鉴定的来自革兰氏阴性土壤细菌Niabella aurantiaca DSM 17617 的 β- N-乙酰半乳糖胺酶。当在大肠杆菌中重组表达时,该酶选择性地裂解的 4-硝基苯基-N-乙酰基-β- d-半乳糖胺 ( p NP-β -d -Gal p NAc)。该蛋白的X射线晶体结构被细化至2.5 Å,由N端β-夹心结构域和(β/α) 8桶催化结构域组成。尽管只有 22% 的序列同一性, Na Nga的 3D 结构与之前为 GH123 家族成员确定的结构相似,表明它也采用相同的底物辅助催化机制。N-乙酰基半乳糖胺噻唑啉(GalNAc-噻唑啉)的抑制支持了所提出的机制。对其近端序列空间的系统发育分析显示,Na Nga 周围的未知序列显着聚类,与先前鉴定的 GH123 成员有足够的分歧,可以将该家族细分为不同的亚家族。尽管我们的酶的实际生物底物仍然未知,但对活性位点袋的检查表明它可能是在 O-3 处被单糖取代的β- N-乙酰半乳糖胺。基因组背景分析反过来表明,这种取代的 β- N-乙酰氨基半乳糖可能附加到来自环境放线菌的d-阿拉伯聚糖上。
更新日期:2023-12-21
中文翻译:

一种来自 Niabella aurantiaca 的新型 β-N-乙酰半乳糖胺酶的表征和结构研究
我们在此报告了Na Nga的鉴定、表征和三维 (3D) 结构测定,Na Nga 是一种新鉴定的来自革兰氏阴性土壤细菌Niabella aurantiaca DSM 17617 的 β- N-乙酰半乳糖胺酶。当在大肠杆菌中重组表达时,该酶选择性地裂解的 4-硝基苯基-N-乙酰基-β- d-半乳糖胺 ( p NP-β -d -Gal p NAc)。该蛋白的X射线晶体结构被细化至2.5 Å,由N端β-夹心结构域和(β/α) 8桶催化结构域组成。尽管只有 22% 的序列同一性, Na Nga的 3D 结构与之前为 GH123 家族成员确定的结构相似,表明它也采用相同的底物辅助催化机制。N-乙酰基半乳糖胺噻唑啉(GalNAc-噻唑啉)的抑制支持了所提出的机制。对其近端序列空间的系统发育分析显示,Na Nga 周围的未知序列显着聚类,与先前鉴定的 GH123 成员有足够的分歧,可以将该家族细分为不同的亚家族。尽管我们的酶的实际生物底物仍然未知,但对活性位点袋的检查表明它可能是在 O-3 处被单糖取代的β- N-乙酰半乳糖胺。基因组背景分析反过来表明,这种取代的 β- N-乙酰氨基半乳糖可能附加到来自环境放线菌的d-阿拉伯聚糖上。



























 京公网安备 11010802027423号
京公网安备 11010802027423号