当前位置:
X-MOL 学术
›
FEBS Journal
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mutations at proximal cysteine residues in PML impair ATO binding by destabilizing the RBCC domain
FEBS Journal Pub Date : 2023-12-21 , DOI: 10.1111/febs.17041 Suchita Dubey 1, 2 , Neha Mishra 1, 2 , Rohan Shelke 1 , Ashok K Varma 1, 2
FEBS Journal Pub Date : 2023-12-21 , DOI: 10.1111/febs.17041 Suchita Dubey 1, 2 , Neha Mishra 1, 2 , Rohan Shelke 1 , Ashok K Varma 1, 2
Affiliation
|
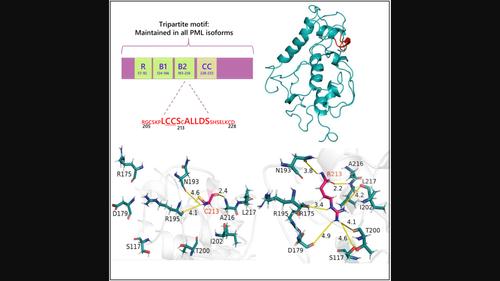
Acute promyelocytic leukemia (APL) is characterized by the fusion gene promyelocytic leukemia–retinoic acid receptor-alpha (PML–RARA) and is conventionally treated with arsenic trioxide (ATO). ATO binds directly to the RING finger, B-box, coiled-coil (RBCC) domain of PML and initiates degradation of the fusion oncoprotein PML–RARA. However, the mutational hotspot at C212–S220 disrupts ATO binding, leading to drug resistance in APL. Therefore, structural consequences of these point mutations in PML that remain uncertain require comprehensive analysis. In this study, we investigated the structure-based ensemble properties of the promyelocytic leukemia-RING-B-box-coiled-coil (PML-RBCC) domains and ATO-resistant mutations. Oligomeric studies reveal that PML-RBCC wild-type and mutants C212R, S214L, A216T, L217F, and S220G predominantly form tetramers, whereas mutants C213R, A216V, L218P, and D219H tend to form dimers. The stability of the dimeric mutants was lower, exhibiting a melting temperature (Tm) reduction of 30 °C compared with the tetrameric mutants and wild-type PML protein. Furthermore, the exposed surface of the C213R mutation rendered it more prone to protease digestion than that of the C212R mutation. The spectroscopic analysis highlighted ATO-induced structural alterations in S214L, A216V, and D219H mutants, in contrast to C213R, L217F, and L218P mutations. Moreover, the computational analysis revealed that the ATO-resistant mutations C213R, A216V, L217F, and L218P caused changes in the size, shape, and flexibility of the PML-RBCC wild-type protein. The mutations C213R, A216V, L217F, and L218P destabilize the wild-type protein structure due to the adaptation of distinct conformational changes. In addition, these mutations disrupt several hydrogen bonds, including interactions involving C212, C213, and C215, which are essential for ATO binding. The local and global structural features induced by these mutations provide mechanistic insight into ATO resistance and APL pathogenesis.
中文翻译:

PML 中近端半胱氨酸残基的突变通过破坏 RBCC 结构域的稳定性来损害 ATO 结合
急性早幼粒细胞白血病(APL)的特征是融合基因早幼粒细胞白血病-视黄酸受体-α(PML - RARA),传统上用三氧化二砷(ATO)治疗。 ATO 直接与 PML 的环指、B 盒、卷曲螺旋 (RBCC) 结构域结合,并启动融合癌蛋白PML – RARA的降解。然而,C212-S220 处的突变热点破坏了 ATO 结合,导致 APL 产生耐药性。因此,PML 中这些点突变的结构后果仍不确定,需要进行全面分析。在这项研究中,我们研究了早幼粒细胞白血病-RING-B-box-coiled-coil (PML-RBCC) 结构域和 ATO 抗性突变的基于结构的整体特性。寡聚研究表明,PML-RBCC 野生型和突变体 C212R、S214L、A216T、L217F 和 S220G 主要形成四聚体,而突变体 C213R、A216V、L218P 和 D219H 倾向于形成二聚体。与四聚体突变体和野生型 PML 蛋白相比,二聚体突变体的稳定性较低,熔解温度 ( T m ) 降低了 30 °C。此外,C213R 突变的暴露表面使其比 C212R 突变更容易被蛋白酶消化。光谱分析强调了 ATO 诱导的 S214L、A216V 和 D219H 突变体的结构改变,与 C213R、L217F 和 L218P 突变形成鲜明对比。此外,计算分析表明,ATO 抗性突变 C213R、A216V、L217F 和 L218P 导致 PML-RBCC 野生型蛋白的大小、形状和灵活性发生变化。由于不同构象变化的适应,突变 C213R、A216V、L217F 和 L218P 破坏了野生型蛋白质结构的稳定性。此外,这些突变还会破坏多个氢键,包括涉及 C212、C213 和 C215 的相互作用,这些氢键对于 ATO 结合至关重要。这些突变引起的局部和整体结构特征为 ATO 抗性和 APL 发病机制提供了机制见解。
更新日期:2023-12-21
中文翻译:

PML 中近端半胱氨酸残基的突变通过破坏 RBCC 结构域的稳定性来损害 ATO 结合
急性早幼粒细胞白血病(APL)的特征是融合基因早幼粒细胞白血病-视黄酸受体-α(PML - RARA),传统上用三氧化二砷(ATO)治疗。 ATO 直接与 PML 的环指、B 盒、卷曲螺旋 (RBCC) 结构域结合,并启动融合癌蛋白PML – RARA的降解。然而,C212-S220 处的突变热点破坏了 ATO 结合,导致 APL 产生耐药性。因此,PML 中这些点突变的结构后果仍不确定,需要进行全面分析。在这项研究中,我们研究了早幼粒细胞白血病-RING-B-box-coiled-coil (PML-RBCC) 结构域和 ATO 抗性突变的基于结构的整体特性。寡聚研究表明,PML-RBCC 野生型和突变体 C212R、S214L、A216T、L217F 和 S220G 主要形成四聚体,而突变体 C213R、A216V、L218P 和 D219H 倾向于形成二聚体。与四聚体突变体和野生型 PML 蛋白相比,二聚体突变体的稳定性较低,熔解温度 ( T m ) 降低了 30 °C。此外,C213R 突变的暴露表面使其比 C212R 突变更容易被蛋白酶消化。光谱分析强调了 ATO 诱导的 S214L、A216V 和 D219H 突变体的结构改变,与 C213R、L217F 和 L218P 突变形成鲜明对比。此外,计算分析表明,ATO 抗性突变 C213R、A216V、L217F 和 L218P 导致 PML-RBCC 野生型蛋白的大小、形状和灵活性发生变化。由于不同构象变化的适应,突变 C213R、A216V、L217F 和 L218P 破坏了野生型蛋白质结构的稳定性。此外,这些突变还会破坏多个氢键,包括涉及 C212、C213 和 C215 的相互作用,这些氢键对于 ATO 结合至关重要。这些突变引起的局部和整体结构特征为 ATO 抗性和 APL 发病机制提供了机制见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号