当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel insight from in-/ex-situ investigation toward intercalated water in high-performance oxygen evolution electrocatalysts and their mechanisms
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2023-12-22 , DOI: 10.1002/jccs.202300359 Guan-Bo Wang, Li-Ling Lin, Hsiang-Ting Wang, Hao Ming Chen
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2023-12-22 , DOI: 10.1002/jccs.202300359 Guan-Bo Wang, Li-Ling Lin, Hsiang-Ting Wang, Hao Ming Chen
|
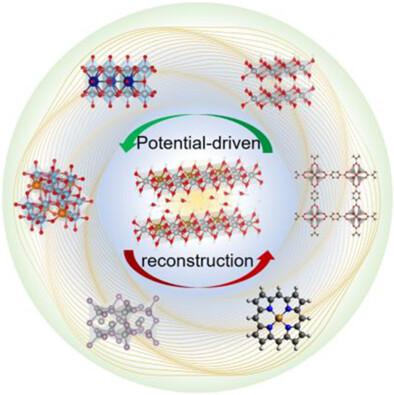
Hydrogen is a green energy source with zero carbon emissions and renewable properties. Green hydrogen, produced via water electrolysis, can efficiently harness excess renewable energy during peak periods, making it a key player in grid stabilization through processes like power-to-gas. Consequently, there is a pressing need to develop catalysts with high activity, stability, and cost-effectiveness in the energy sector. The development of electrochemical (EC) water splitting, a promising path to meet alternative energy demands, however, is hindered by two main challenges that persist in water splitting: the anodic oxygen evolution reaction (OER) catalysts still need lower overpotential, and they must have enough stability with high catalytic activity. Both factors determine water electrolysis reactions' overall energy consumption and commercialization potential. This article introduces several significant parameters for assessing catalyst performance; three primary OER mechanisms are also briefly reviewed. Thereby, numerous electrocatalysts for OER are categorized by their composition and morphology. Furthermore, we generalize a common phenomenon that occurs at the surface of catalysts during the OER process. It is deduced that the surface transformation from as-prepared to an activated state, that is, the surface-environment change of the active site due to redox-induced dissolution and re-deposition, plays a critical role in OER. On the other hand, generating a layered structure leads to the accommodation of intercalated water molecules, which may enhance the activity and robustness via hydrogen bonds and dominate the absorption energy of oxygen species on the metal site. Lastly, we enumerate several in-/ex-situ methodologies that can discriminate the real active sites of the bulk, near-surface region, interface, and intermediates adsorbed on the electrocatalyst surface. Further investigation is needed to unveil the interaction between active sites and embedded water molecules in EC catalytic processes. This paper provides a novel perspective of the intercalated water for future development of OER electrocatalysts, simultaneously considering performance and stability.
中文翻译:

对高性能析氧电催化剂中插层水及其机理的原位/异位研究的新见解
氢是一种具有零碳排放和可再生特性的绿色能源。通过水电解产生的绿色氢气可以在高峰时段有效利用多余的可再生能源,使其成为通过电转气等过程稳定电网的关键角色。因此,能源领域迫切需要开发具有高活性、稳定性和成本效益的催化剂。电化学(EC)水分解是满足替代能源需求的一条有前途的途径,然而,水分解中持续存在的两个主要挑战阻碍了其发展:阳极析氧反应(OER)催化剂仍然需要较低的过电势,并且它们必须具有足够的稳定性和较高的催化活性。这两个因素决定了水电解反应的总体能耗和商业化潜力。本文介绍了评估催化剂性能的几个重要参数;还简要回顾了三种主要的开放教育资源机制。因此,许多 OER 电催化剂根据其组成和形态进行分类。此外,我们概括了 OER 过程中催化剂表面发生的常见现象。据推测,从制备状态到活化状态的表面转变,即由于氧化还原诱导的溶解和再沉积而导致的活性位点的表面环境变化,在OER中起着关键作用。另一方面,产生层状结构导致嵌入水分子的容纳,这可以通过氢键增强活性和鲁棒性,并主导金属位点上氧物质的吸收能。最后,我们列举了几种可以区分电催化剂表面吸附的本体、近表面区域、界面和中间体的真实活性位点的原位/异位方法。需要进一步研究来揭示 EC 催化过程中活性位点和嵌入水分子之间的相互作用。本文为 OER 电催化剂的未来发展提供了插层水的新视角,同时考虑了性能和稳定性。
更新日期:2023-12-22
中文翻译:

对高性能析氧电催化剂中插层水及其机理的原位/异位研究的新见解
氢是一种具有零碳排放和可再生特性的绿色能源。通过水电解产生的绿色氢气可以在高峰时段有效利用多余的可再生能源,使其成为通过电转气等过程稳定电网的关键角色。因此,能源领域迫切需要开发具有高活性、稳定性和成本效益的催化剂。电化学(EC)水分解是满足替代能源需求的一条有前途的途径,然而,水分解中持续存在的两个主要挑战阻碍了其发展:阳极析氧反应(OER)催化剂仍然需要较低的过电势,并且它们必须具有足够的稳定性和较高的催化活性。这两个因素决定了水电解反应的总体能耗和商业化潜力。本文介绍了评估催化剂性能的几个重要参数;还简要回顾了三种主要的开放教育资源机制。因此,许多 OER 电催化剂根据其组成和形态进行分类。此外,我们概括了 OER 过程中催化剂表面发生的常见现象。据推测,从制备状态到活化状态的表面转变,即由于氧化还原诱导的溶解和再沉积而导致的活性位点的表面环境变化,在OER中起着关键作用。另一方面,产生层状结构导致嵌入水分子的容纳,这可以通过氢键增强活性和鲁棒性,并主导金属位点上氧物质的吸收能。最后,我们列举了几种可以区分电催化剂表面吸附的本体、近表面区域、界面和中间体的真实活性位点的原位/异位方法。需要进一步研究来揭示 EC 催化过程中活性位点和嵌入水分子之间的相互作用。本文为 OER 电催化剂的未来发展提供了插层水的新视角,同时考虑了性能和稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号