当前位置:
X-MOL 学术
›
J. Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting Rab-RILPL interactions as a strategy to downregulate pathogenic LRRK2 in Parkinson's disease
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2023-12-22 , DOI: 10.1002/psc.3563 Krista K. Alexander 1 , Yahaira Naaldijk 2 , Rachel Fasiczka 2 , Besma Brahmia 2 , Tiancheng Chen 1 , Sabine Hilfiker 2 , Eileen J. Kennedy 1
Journal of Peptide Science ( IF 2.1 ) Pub Date : 2023-12-22 , DOI: 10.1002/psc.3563 Krista K. Alexander 1 , Yahaira Naaldijk 2 , Rachel Fasiczka 2 , Besma Brahmia 2 , Tiancheng Chen 1 , Sabine Hilfiker 2 , Eileen J. Kennedy 1
Affiliation
|
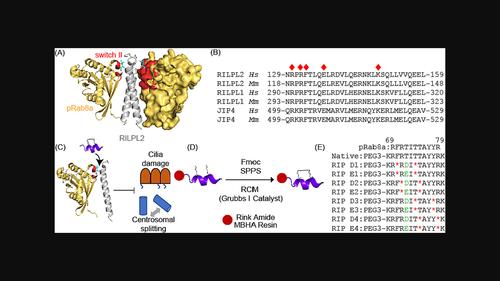
Familial Parkinson's disease (PD) is frequently linked to multiple disease-causing mutations within Leucine-Rich Repeat Protein Kinase 2 (LRRK2), leading to aberrant kinase activity. Multiple pathogenic effects of enhanced LRRK2 activity have been identified, including loss of cilia and centrosomal cohesion defects. When phosphorylated by LRRK2, Rab8a and Rab10 bind to phospho-specific RILPL effector proteins. RILPL-mediated accumulation of pRabs proximal to the mother centriole is critical for initiating deficits in ciliogenesis and centrosome cohesion mediated by LRRK2. We hypothesized that Rab-derived phospho-mimics may serve to block phosphorylated Rab proteins from docking with RILPL in the context of hyperactive LRRK2 mutants. This would serve as an alternative strategy to downregulate pathogenic signaling mediated by LRRK2, rather than targeting LRRK2 kinase activity itself. To test this theory, we designed a series of constrained peptides mimicking phosphorylated Switch II derived from Rab8. These RILPL interacting peptides, termed RIP, were further shown to permeate cells. Further, several peptides were found to bind RILPL2 and restore ciliogenesis and centrosomal cohesion defects in cells expressing PD-associated mutant LRRK2. This research demonstrates the utility of constrained peptides as downstream inhibitors to target pathogenic LRRK2 activity and may provide an alternative approach to target specific pathways activated by LRRK2.
中文翻译:

靶向 Rab-RILPL 相互作用作为下调帕金森病致病性 LRRK2 的策略
家族性帕金森病 (PD) 通常与富含亮氨酸重复蛋白激酶 2 (LRRK2) 内的多种致病突变有关,从而导致激酶活性异常。 LRRK2 活性增强的多种致病作用已被确定,包括纤毛丧失和中心体凝聚力缺陷。当 LRRK2 磷酸化时,Rab8a 和 Rab10 与磷酸特异性 RILPL 效应蛋白结合。 RILPL 介导的 pRab 靠近母体中心粒的积累对于 LRRK2 介导的纤毛发生和中心体凝聚力的启动缺陷至关重要。我们假设,在高度活跃的 LRRK2 突变体中,Rab 衍生的磷酸模拟物可能有助于阻止磷酸化 Rab 蛋白与 RILPL 对接。这将作为下调 LRRK2 介导的致病信号传导的替代策略,而不是针对 LRRK2 激酶活性本身。为了验证这一理论,我们设计了一系列模仿源自 Rab8 的磷酸化 Switch II 的受限肽。这些 RILPL 相互作用肽(称为 RIP)被进一步证明可以渗透细胞。此外,还发现几种肽可以结合 RILPL2,并恢复表达 PD 相关突变体 LRRK2 的细胞中的纤毛发生和中心体凝聚缺陷。这项研究证明了限制肽作为下游抑制剂来靶向致病性 LRRK2 活性的效用,并可能提供一种替代方法来靶向 LRRK2 激活的特定途径。
更新日期:2023-12-22
中文翻译:

靶向 Rab-RILPL 相互作用作为下调帕金森病致病性 LRRK2 的策略
家族性帕金森病 (PD) 通常与富含亮氨酸重复蛋白激酶 2 (LRRK2) 内的多种致病突变有关,从而导致激酶活性异常。 LRRK2 活性增强的多种致病作用已被确定,包括纤毛丧失和中心体凝聚力缺陷。当 LRRK2 磷酸化时,Rab8a 和 Rab10 与磷酸特异性 RILPL 效应蛋白结合。 RILPL 介导的 pRab 靠近母体中心粒的积累对于 LRRK2 介导的纤毛发生和中心体凝聚力的启动缺陷至关重要。我们假设,在高度活跃的 LRRK2 突变体中,Rab 衍生的磷酸模拟物可能有助于阻止磷酸化 Rab 蛋白与 RILPL 对接。这将作为下调 LRRK2 介导的致病信号传导的替代策略,而不是针对 LRRK2 激酶活性本身。为了验证这一理论,我们设计了一系列模仿源自 Rab8 的磷酸化 Switch II 的受限肽。这些 RILPL 相互作用肽(称为 RIP)被进一步证明可以渗透细胞。此外,还发现几种肽可以结合 RILPL2,并恢复表达 PD 相关突变体 LRRK2 的细胞中的纤毛发生和中心体凝聚缺陷。这项研究证明了限制肽作为下游抑制剂来靶向致病性 LRRK2 活性的效用,并可能提供一种替代方法来靶向 LRRK2 激活的特定途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号