当前位置:
X-MOL 学术
›
Mol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coexpressedδ-,μ-, andκ-Opioid Receptors Modulate Voltage-Gated Ca2+Channels in Gastric-Projecting Vagal Afferent Neurons
Molecular Pharmacology ( IF 3.6 ) Pub Date : 2024-03-01 , DOI: 10.1124/molpharm.123.000774 Hannah J. Goudsward , Victor Ruiz-Velasco , Salvatore L. Stella , Lisa B. Willing , Gregory M. Holmes
Molecular Pharmacology ( IF 3.6 ) Pub Date : 2024-03-01 , DOI: 10.1124/molpharm.123.000774 Hannah J. Goudsward , Victor Ruiz-Velasco , Salvatore L. Stella , Lisa B. Willing , Gregory M. Holmes
|
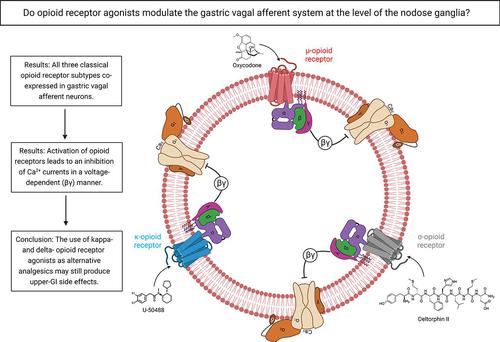
Opioid analgesics are frequently associated with gastrointestinal side effects, including constipation, nausea, dysphagia, and reduced gastric motility. Though it has been shown that stimulation of opioid receptors expressed in enteric motor neurons contributes to opioid-induced constipation, it remains unclear whether activation of opioid receptors in gastric-projecting nodose ganglia neurons contributes to the reduction in gastric motility and emptying associated with opioid use. In the present study, whole-cell patch-clamp recordings were performed to determine the mechanism underlying opioid receptor–mediated modulation of Ca2+ currents in acutely isolated gastric vagal afferent neurons. Our results demonstrate that CaV2.2 channels provide the majority (71% ± 16%) of Ca2+ currents in gastric vagal afferent neurons. Furthermore, we found that application of oxycodone, U-50488, or deltorphin II on gastric nodose ganglia neurons inhibited Ca2+ currents through a voltage-dependent mechanism by coupling to the Gαi/o family of heterotrimeric G-proteins. Because previous studies have demonstrated that the nodose ganglia expresses low levels of δ-opioid receptors, we also determined the deltorphin II concentration-response relationship and assessed deltorphin-mediated Ca2+ current inhibition following exposure to the δ-opioid receptor antagonist ICI 174,864 (0.3 µM). The peak mean Ca2+ current inhibition following deltorphin II application was 47% ± 24% (EC50 = 302.6 nM), and exposure to ICI 174,864 blocked deltorphin II–mediated Ca2+ current inhibition (4% ± 4% versus 37% ± 20%). Together, our results suggest that analgesics targeting any opioid receptor subtype can modulate gastric vagal circuits.
中文翻译:

共表达 δ-、μ- 和 κ-阿片受体调节胃投射迷走神经传入神经元的电压门控 Ca2+ 通道
阿片类镇痛药经常与胃肠道副作用相关,包括便秘、恶心、吞咽困难和胃动力减弱。尽管已经证明刺激肠道运动神经元中表达的阿片受体会导致阿片类药物引起的便秘,但尚不清楚胃投射结节神经元中阿片类受体的激活是否会导致与阿片类药物使用相关的胃动力和排空减少。在本研究中,进行全细胞膜片钳记录以确定阿片受体介导的急性分离胃迷走神经传入神经元中Ca 2+电流调节的机制。我们的结果表明,Ca V 2.2 通道提供胃迷走神经传入神经元中大部分 (71% ± 16%) Ca 2+电流。此外,我们发现,在胃结状神经节神经元上应用羟考酮、U-50488 或 deltorphin II,通过与异三聚体 G 蛋白的 G α i/o家族偶联,通过电压依赖性机制抑制 Ca 2+电流。由于先前的研究已证明结状神经节表达低水平的δ -阿片受体,因此我们还确定了 deltorphin II 浓度-反应关系,并评估了暴露于δ -阿片受体拮抗剂 ICI 174,864 后 deltorphin 介导的 Ca 2+电流抑制( 0.3 µM)。使用 deltorphin II 后的峰值平均 Ca 2+电流抑制为 47% ± 24% (EC 50 = 302.6 nM),暴露于 ICI 174,864 阻断了 deltorphin II 介导的 Ca 2+电流抑制(4% ± 4% 与 37% ±20%)。总之,我们的结果表明,针对任何阿片受体亚型的镇痛药都可以调节胃迷走神经回路。
更新日期:2024-02-15
中文翻译:

共表达 δ-、μ- 和 κ-阿片受体调节胃投射迷走神经传入神经元的电压门控 Ca2+ 通道
阿片类镇痛药经常与胃肠道副作用相关,包括便秘、恶心、吞咽困难和胃动力减弱。尽管已经证明刺激肠道运动神经元中表达的阿片受体会导致阿片类药物引起的便秘,但尚不清楚胃投射结节神经元中阿片类受体的激活是否会导致与阿片类药物使用相关的胃动力和排空减少。在本研究中,进行全细胞膜片钳记录以确定阿片受体介导的急性分离胃迷走神经传入神经元中Ca 2+电流调节的机制。我们的结果表明,Ca V 2.2 通道提供胃迷走神经传入神经元中大部分 (71% ± 16%) Ca 2+电流。此外,我们发现,在胃结状神经节神经元上应用羟考酮、U-50488 或 deltorphin II,通过与异三聚体 G 蛋白的 G α i/o家族偶联,通过电压依赖性机制抑制 Ca 2+电流。由于先前的研究已证明结状神经节表达低水平的δ -阿片受体,因此我们还确定了 deltorphin II 浓度-反应关系,并评估了暴露于δ -阿片受体拮抗剂 ICI 174,864 后 deltorphin 介导的 Ca 2+电流抑制( 0.3 µM)。使用 deltorphin II 后的峰值平均 Ca 2+电流抑制为 47% ± 24% (EC 50 = 302.6 nM),暴露于 ICI 174,864 阻断了 deltorphin II 介导的 Ca 2+电流抑制(4% ± 4% 与 37% ±20%)。总之,我们的结果表明,针对任何阿片受体亚型的镇痛药都可以调节胃迷走神经回路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号