当前位置:
X-MOL 学术
›
Cell. Mol. Gastroenterol. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Protease Domain in HEV pORF1 Mediates the Replicase’s Localization to Multivesicular Bodies and Its Exosomal Release
Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2024-01-06 , DOI: 10.1016/j.jcmgh.2024.01.001 Mirco Glitscher , Inga Mareike Spannaus , Fabiane Behr , Robin Oliver Murra , Kathrin Woytinek , Daniela Bender , Eberhard Hildt
Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2024-01-06 , DOI: 10.1016/j.jcmgh.2024.01.001 Mirco Glitscher , Inga Mareike Spannaus , Fabiane Behr , Robin Oliver Murra , Kathrin Woytinek , Daniela Bender , Eberhard Hildt
|
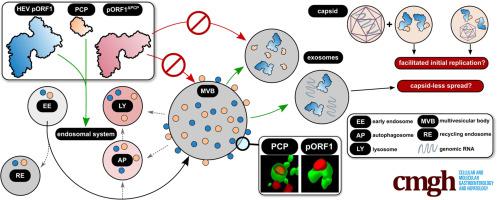
A peculiar feature of the hepatitis E virus (HEV) is its reliance on the exosomal route for viral release. Genomic replication is mediated via the viral polyprotein pORF1, yet little is known about its subcellular localization. Subcellular localization of pORF1 and its subdomains, generated and cloned based on a structural prediciton of the viral replicase, was analyzed via confocal laser scanning microscopy. Exosomes released from cells were isolated via ultracentrifugation and analyzed by isopycnic density gradient centrifugation. This was followed by fluorimetry or Western blot analyses or reverse transcriptase–polymerase chain reaction to analyze separated particles in more detail. We found pORF1 to be accumulating within the endosomal system, most dominantly to multivesicular bodies (MVBs). Expression of the polyprotein’s 7 subdomains revealed that the papain-like cysteine-protease (PCP) is the only domain localizing like the full-length protein. A PCP-deficient pORF1 mutant lost its association to MVBs. Strikingly, both pORF1 and PCP can be released via exosomes. Similarly, genomic RNA still is released via exosomes in the absence of pORF2/3. Taken together, we found that pORF1 localizes to MVBs in a PCP-dependent manner, which is followed by exosomal release. This reveals new aspects of HEV life cycle, because replication and release could be coupled at the endosomal interface. In addition, this may mediate capsid-independent spread or may facilitate the spread of viral infection, because genomes entering the cell during de novo infection readily encounter exosomally transferred pORF1.
中文翻译:

HEV pORF1 中的蛋白酶结构域介导复制酶对多泡体的定位及其外泌体释放
戊型肝炎病毒(HEV)的一个独特特征是其依赖外泌体途径释放病毒。基因组复制是通过病毒多蛋白 pORF1 介导的,但对其亚细胞定位知之甚少。通过共聚焦激光扫描显微镜分析了基于病毒复制酶的结构预测生成和克隆的 pORF1 及其子结构域的亚细胞定位。通过超速离心分离从细胞释放的外泌体,并通过等密度梯度离心进行分析。随后进行荧光测定或蛋白质印迹分析或逆转录酶-聚合酶链反应,以更详细地分析分离的颗粒。我们发现 pORF1 在内体系统内积累,主要是多泡体 (MVB)。多蛋白的 7 个子结构域的表达表明,木瓜蛋白酶样半胱氨酸蛋白酶 (PCP) 是唯一像全长蛋白一样定位的结构域。 PCP 缺陷的 pORF1 突变体失去了与 MVB 的关联。引人注目的是,pORF1 和 PCP 都可以通过外泌体释放。同样,在 pORF2/3 不存在的情况下,基因组 RNA 仍然通过外泌体释放。综上所述,我们发现 pORF1 以 PCP 依赖性方式定位到 MVB,随后释放外泌体。这揭示了 HEV 生命周期的新方面,因为复制和释放可以在内体界面耦合。此外,这可能介导不依赖衣壳的传播或可能促进病毒感染的传播,因为在从头感染期间进入细胞的基因组很容易遇到外泌体转移的pORF1。
更新日期:2024-01-06
中文翻译:

HEV pORF1 中的蛋白酶结构域介导复制酶对多泡体的定位及其外泌体释放
戊型肝炎病毒(HEV)的一个独特特征是其依赖外泌体途径释放病毒。基因组复制是通过病毒多蛋白 pORF1 介导的,但对其亚细胞定位知之甚少。通过共聚焦激光扫描显微镜分析了基于病毒复制酶的结构预测生成和克隆的 pORF1 及其子结构域的亚细胞定位。通过超速离心分离从细胞释放的外泌体,并通过等密度梯度离心进行分析。随后进行荧光测定或蛋白质印迹分析或逆转录酶-聚合酶链反应,以更详细地分析分离的颗粒。我们发现 pORF1 在内体系统内积累,主要是多泡体 (MVB)。多蛋白的 7 个子结构域的表达表明,木瓜蛋白酶样半胱氨酸蛋白酶 (PCP) 是唯一像全长蛋白一样定位的结构域。 PCP 缺陷的 pORF1 突变体失去了与 MVB 的关联。引人注目的是,pORF1 和 PCP 都可以通过外泌体释放。同样,在 pORF2/3 不存在的情况下,基因组 RNA 仍然通过外泌体释放。综上所述,我们发现 pORF1 以 PCP 依赖性方式定位到 MVB,随后释放外泌体。这揭示了 HEV 生命周期的新方面,因为复制和释放可以在内体界面耦合。此外,这可能介导不依赖衣壳的传播或可能促进病毒感染的传播,因为在从头感染期间进入细胞的基因组很容易遇到外泌体转移的pORF1。



























 京公网安备 11010802027423号
京公网安备 11010802027423号