NanoImpact ( IF 4.9 ) Pub Date : 2024-01-07 , DOI: 10.1016/j.impact.2024.100492 Zhaoxun Yang , Jean-François Gaillard
|
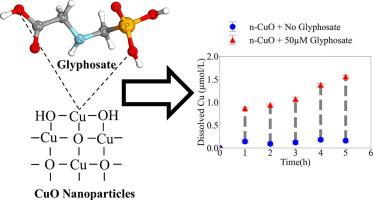
Recently CuO nanoparticles (n-CuO) have been proposed as an alternative method to deliver a Cu-based pesticide for controlling fungal infestations. With the concomitant use of glyphosate as an herbicide, the interactions between n-CuO and this strong ligand need to be assessed. We investigated the dissolution kinetics of n-CuO and bulk-CuO (b-CuO) particles in the presence of a commercial glyphosate product and compared it to oxalate, a natural ligand present in soil water. We performed experiments at concentration levels representative of the conditions under which n-CuO and glyphosate would be used (∼0.9 mg/L n-CuO and 50 μM of glyphosate). As tenorite (CuO) dissolution kinetics are known to be surface controlled, we determined that at pH 6.5, T ∼ 20 °C, using KNO3 as background electrolyte, the presence of glyphosate leads to a dissolution rate of 9.3 ± 0.7 10−3 h−1. In contrast, in absence of glyphosate, and under the same conditions, it is 2 orders of magnitude less: 8.9 ± 3.6 10−5 h−1. In a more complex multi-electrolyte aqueous solution the same effect is observed; glyphosate promotes the dissolution rates of n-CuO and b-CuO within the first 10 h of reaction by a factor of ∼2 to ∼15. In the simple KNO3 electrolyte, oxalate leads to dissolution rates of CuO about two times faster than glyphosate. However, the kinetic rates within the first 10 h of reaction are about the same for the two ligands when the reaction takes place in the multi-electrolyte solution as oxalate is mostly bound to Ca2+ and Mg2+.
中文翻译:

草甘膦存在下氧化铜纳米粒子的溶解动力学
最近,有人提出使用 CuO 纳米粒子(n-CuO)作为一种替代方法来提供铜基农药来控制真菌感染。与草甘膦作为除草剂同时使用时,需要评估 n-CuO 和这种强配体之间的相互作用。我们研究了在商业草甘膦产品存在下 n-CuO 和块状 CuO (b-CuO) 颗粒的溶解动力学,并将其与草酸盐(土壤水中存在的天然配体)进行比较。我们在代表 n-CuO 和草甘膦使用条件的浓度水平(~0.9 mg/L n-CuO 和 50 μM 草甘膦)进行了实验。由于已知黑长石 (CuO) 溶解动力学受表面控制,我们确定在 pH 6.5、T ∼ 20 °C 下,使用 KNO 3作为背景电解质,草甘膦的存在导致溶解速率为 9.3 ± 0.710 -3 小时-1。相比之下,在没有草甘膦的情况下,在相同条件下,它要低 2 个数量级:8.9 ± 3.610 -5 小时-1。在更复杂的多电解质水溶液中观察到相同的效果;草甘膦在反应的前 10 小时内将 n-CuO 和 b-CuO 的溶解速率提高约 2 至约 15 倍。在简单的 KNO 3电解质中,草酸盐导致 CuO 的溶解速度比草甘膦快大约两倍。然而,当反应在多电解质溶液中发生时,两个配体在反应的前10小时内的动力学速率大致相同,因为草酸盐主要与Ca 2+和Mg 2+结合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号