Journal of Loss Prevention in the Process Industries ( IF 3.5 ) Pub Date : 2024-01-06 , DOI: 10.1016/j.jlp.2024.105245 Dongjin Yu , Wang Sun , Peng Li , Jie Hu , Dayong Jiang , Zhenyun Wei , Chunsheng Cheng , Xiaolei Wang , Lizhi Liu
|
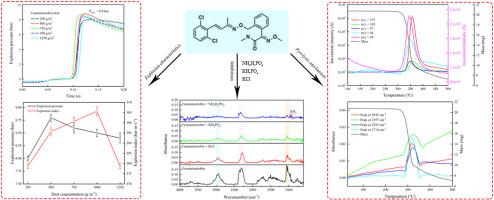
In this study, the explosion and pyrolysis characteristics of fungicide fenaminstrobin dust were studied, and the inhibitory effects of three explosion suppressants, namely, NH4H2PO4, KH2PO4, and KCl, on dust explosion were compared. A combined thermogravimetric (TG)–Fourier transform infrared (FTIR)–mass spectrometry (MS) technique was adopted to analyze the inhibition mechanism. The results showed that the minimum ignition energy (MIE) was 5 mJ, the explosion index (Kst) was 305.7 bar m/s, and the dust explosion hazard was severe. We found that all three compounds (NH4H2PO4, KH2PO4, and KCl) could be used as explosion suppressants. When the addition dose of NH4H2PO4 was 35%, the Kst value dropped by 40.7%, and MIE exceeded 1000 mJ. When the addition of KH2PO4 was 50%, the Kst value dropped by 37.3%, and the MIE increased to 300 mJ. In comparison, NH4H2PO4 showed the best inhibitory effect, while KCl demonstrated the worst inhibitory effect. The pyrolysis of fenaminstrobin produced organic gases, including quinoline, benzonitrile, and methyl isocyanate, and generated large quantities of free radicals. The explosion suppressants could inhibit the explosion through physical pathways (endothermic, coating, and oxygen-diluting processes) and chemical pathways (free-radical capture).
中文翻译:

抑爆剂对杀菌剂非那明肟酯粉尘爆炸及热解特性的影响
研究了杀菌剂芬胺肟酯粉尘的爆炸和热解特性,比较了NH 4 H 2 PO 4、KH 2 PO 4和KCl 3种抑爆剂对粉尘爆炸的抑制效果。采用热重(TG)-傅里叶变换红外(FTIR)-质谱(MS)联合技术分析抑制机制。结果表明,最小点火能量(MIE)为5 mJ,爆炸指数(K st)为305.7 bar m/s,粉尘爆炸危险严重。我们发现所有三种化合物(NH 4 H 2 PO 4、KH 2 PO 4和KCl)都可以用作爆炸抑制剂。当NH 4 H 2 PO 4添加剂量为35%时,K st值下降40.7%,MIE超过1000 mJ。当KH 2 PO 4的添加量为50%时,K st值下降了37.3%,MIE增加至300 mJ。相比之下,NH 4 H 2 PO 4的抑制效果最好,而KCl的抑制效果最差。芬那肟酯热解产生有机气体,包括喹啉、苯甲腈和异氰酸甲酯,并产生大量自由基。爆炸抑制剂可以通过物理途径(吸热、涂层和氧气稀释过程)和化学途径(自由基捕获)抑制爆炸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号