当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anaerobic photocyclization of ortho-dialkylamino substituted stiff-stilbenes
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2024-01-08 , DOI: 10.1002/jccs.202300427 Yi-Ning Hung, Yi-Ching Wang, Zi-Jian Chen, Jye-Shane Yang
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2024-01-08 , DOI: 10.1002/jccs.202300427 Yi-Ning Hung, Yi-Ching Wang, Zi-Jian Chen, Jye-Shane Yang
|
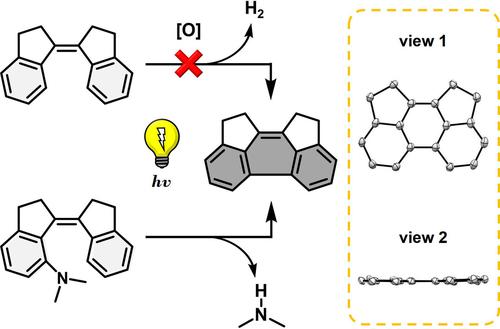
The photocyclization of diarylethenes toward polycyclic aromatic hydrocarbons and analogs has been well-documented. However, stiff-stilbene was reported to be inactive, and examples of photocyclization-induced deamination remain unknown. Here, we report that ortho-(N,N-dimethylamino)-stiff-stilbene (1a) undergoes efficient photocyclization-induced deamination, forming the dicyclopenta-fused phenanthrene (DCPP) with good yield (65%) under anaerobic conditions. The relative reactivities of stiff-stilbenes with different o-amino substituents (1b–1g) or with an o-methoxy (1h) group, and those of o-(N,N-dimethylamino)stilbenes (2a and 2aMe), provide insights into the substituent and structural effects.
中文翻译:

邻位二烷基氨基取代的二苯乙烯的厌氧光环化
二芳基乙烯光环化生成多环芳烃及其类似物已有充分记录。然而,据报道,刚性二苯乙烯是无活性的,并且光环化诱导的脱氨基的例子仍然未知。在这里,我们报道了邻-( N,N-二甲基氨基)-硬-二苯乙烯( 1a )在厌氧条件下经历了有效的光环化诱导脱氨,形成双环五稠合菲( DCPP ),产率良好(65%)。具有不同邻氨基取代基 ( 1b–1g ) 或邻甲氧基 ( 1h ) 基团的硬二苯乙烯的相对反应性,以及邻-( N,N-二甲基氨基)二苯乙烯(2a和2aMe)的相对反应性,提供了见解分为取代基和结构效应。
更新日期:2024-01-08
中文翻译:

邻位二烷基氨基取代的二苯乙烯的厌氧光环化
二芳基乙烯光环化生成多环芳烃及其类似物已有充分记录。然而,据报道,刚性二苯乙烯是无活性的,并且光环化诱导的脱氨基的例子仍然未知。在这里,我们报道了邻-( N,N-二甲基氨基)-硬-二苯乙烯( 1a )在厌氧条件下经历了有效的光环化诱导脱氨,形成双环五稠合菲( DCPP ),产率良好(65%)。具有不同邻氨基取代基 ( 1b–1g ) 或邻甲氧基 ( 1h ) 基团的硬二苯乙烯的相对反应性,以及邻-( N,N-二甲基氨基)二苯乙烯(2a和2aMe)的相对反应性,提供了见解分为取代基和结构效应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号