当前位置:
X-MOL 学术
›
J. Raman Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Characterization of carfentanil and thiofentanil using surface-enhanced Raman spectroscopy and density functional theory
Journal of Raman Spectroscopy ( IF 2.5 ) Pub Date : 2024-01-10 , DOI: 10.1002/jrs.6643 Rasmus Öberg 1, 2 , Lars Landström 3 , Eduardo Gracia‐Espino 1 , Andreas Larsson 2 , Magnus Andersson 1 , Per Ola Andersson 2
Journal of Raman Spectroscopy ( IF 2.5 ) Pub Date : 2024-01-10 , DOI: 10.1002/jrs.6643 Rasmus Öberg 1, 2 , Lars Landström 3 , Eduardo Gracia‐Espino 1 , Andreas Larsson 2 , Magnus Andersson 1 , Per Ola Andersson 2
Affiliation
|
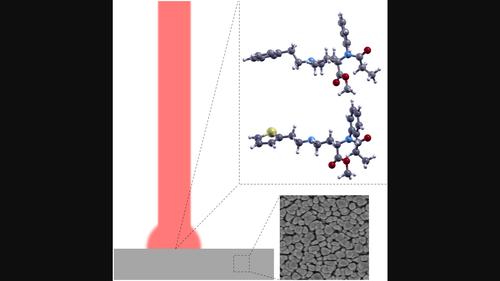
Fentanyls are synthetic opioids up to 10,000 times more potent than morphine. Although initially developed for medical applications, fentanyl and its analogues have recently grown synonymous with the ongoing opioid epidemic. To combat the continued spread of these substances, there is a need for rapid and sensitive techniques for chemical detection. Surface-enhanced Raman spectroscopy (SERS) has the potential for trace detection of harmful chemical substances. However, vibrational spectra obtained by SERS often differ between SERS substrates, as well as compared with spectra from normal Raman (NR) spectroscopy. Herein, SERS and NR responses from two fentanyl analogues, carfentanil (CF) and thiofentanil (TF), were measured and analysed with support from density functional theory (DFT) modelling. Using commercially available silver nanopillar SERS substrates, the SERS signatures of samples diluted in acetonitrile between 0.01 and 1000 µg/mL were studied. Relative SERS peak intensities measured in the range of 220–1800 cm−1 vary with concentration, while SERS and NR spectra largely agree for CF at higher concentrations (
100 µg/mL). For TF, three distinct NR peaks at 262, 366 and 667 cm−1 are absent or strongly suppressed in the SERS spectrum, attributed to the lone-pair electrons of the thiophene's sulphur atom binding to the Ag surface. The concentration dependence of the Raman peak at 1000 cm−1, assigned to trigonal bending of the phenyl ring, approximately follows a Langmuir adsorption isotherm. This work elucidates similarities and differences between SERS and NR in fentanyl detection and discusses the chemical rationale behind these differences.
中文翻译:

使用表面增强拉曼光谱和密度泛函理论表征卡芬太尼和硫芬太尼
芬太尼是合成阿片类药物,其效力比吗啡强 10,000 倍。尽管芬太尼及其类似物最初是为医疗应用而开发的,但最近已成为当前阿片类药物流行的代名词。为了对抗这些物质的持续传播,需要快速、灵敏的化学检测技术。表面增强拉曼光谱(SERS)具有痕量检测有害化学物质的潜力。然而,通过 SERS 获得的振动光谱通常在不同的 SERS 基底之间有所不同,并且与普通拉曼 (NR) 光谱的光谱相比也是如此。在此,在密度泛函理论 (DFT) 模型的支持下,测量和分析了两种芬太尼类似物卡芬太尼 (CF) 和硫芬太尼 (TF) 的 SERS 和 NR 响应。使用市售的银纳米柱 SERS 基底,研究了在乙腈中稀释至 0.01 至 1000 µg/mL 的样品的 SERS 特征。在 220–1800 cm -1范围内测量的相对 SERS 峰强度随浓度变化,而 CF 在较高浓度下的 SERS 和 NR 光谱在很大程度上一致( 100 微克/毫升)。对于TF,SERS光谱中262、366和667 cm -1处的三个不同的NR峰不存在或被强烈抑制,这归因于与Ag表面结合的噻吩硫原子的孤对电子。拉曼峰的浓度依赖性1000 cm -1,指定为苯环的三角弯曲,大致遵循朗缪尔吸附等温线。这项工作阐明了 SERS 和 NR 在芬太尼检测中的异同,并讨论了这些差异背后的化学原理。
更新日期:2024-01-10
中文翻译:

使用表面增强拉曼光谱和密度泛函理论表征卡芬太尼和硫芬太尼
芬太尼是合成阿片类药物,其效力比吗啡强 10,000 倍。尽管芬太尼及其类似物最初是为医疗应用而开发的,但最近已成为当前阿片类药物流行的代名词。为了对抗这些物质的持续传播,需要快速、灵敏的化学检测技术。表面增强拉曼光谱(SERS)具有痕量检测有害化学物质的潜力。然而,通过 SERS 获得的振动光谱通常在不同的 SERS 基底之间有所不同,并且与普通拉曼 (NR) 光谱的光谱相比也是如此。在此,在密度泛函理论 (DFT) 模型的支持下,测量和分析了两种芬太尼类似物卡芬太尼 (CF) 和硫芬太尼 (TF) 的 SERS 和 NR 响应。使用市售的银纳米柱 SERS 基底,研究了在乙腈中稀释至 0.01 至 1000 µg/mL 的样品的 SERS 特征。在 220–1800 cm -1范围内测量的相对 SERS 峰强度随浓度变化,而 CF 在较高浓度下的 SERS 和 NR 光谱在很大程度上一致( 100 微克/毫升)。对于TF,SERS光谱中262、366和667 cm -1处的三个不同的NR峰不存在或被强烈抑制,这归因于与Ag表面结合的噻吩硫原子的孤对电子。拉曼峰的浓度依赖性1000 cm -1,指定为苯环的三角弯曲,大致遵循朗缪尔吸附等温线。这项工作阐明了 SERS 和 NR 在芬太尼检测中的异同,并讨论了这些差异背后的化学原理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号