当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The interaction between NLRP1 and oxidized TRX1 involves a transient disulfide bond
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2024-01-11 , DOI: 10.1016/j.chembiol.2023.12.012 Michael B. Geeson , Jeffrey C. Hsiao , Lydia P. Tsamouri , Daniel P. Ball , Daniel A. Bachovchin
Cell Chemical Biology ( IF 8.6 ) Pub Date : 2024-01-11 , DOI: 10.1016/j.chembiol.2023.12.012 Michael B. Geeson , Jeffrey C. Hsiao , Lydia P. Tsamouri , Daniel P. Ball , Daniel A. Bachovchin
|
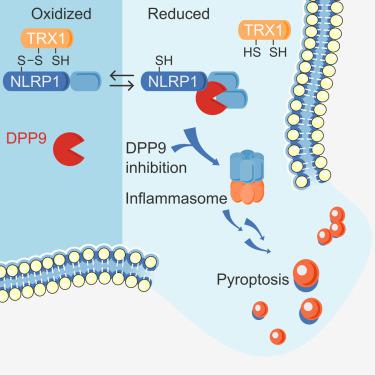
NLRP1 is an innate immune receptor that detects pathogen-associated signals, assembles into a multiprotein structure called an inflammasome, and triggers a proinflammatory form of cell death called pyroptosis. We previously discovered that the oxidized, but not the reduced, form of thioredoxin-1 directly binds to NLRP1 and represses inflammasome formation. However, the molecular basis for NLRP1’s selective association with only the oxidized form of TRX1 has not yet been established. Here, we leveraged AlphaFold-Multimer, site-directed mutagenesis, thiol-trapping experiments, and mass spectrometry to reveal that a specific cysteine residue (C427 in humans) on NLRP1 forms a transient disulfide bond with oxidized TRX1. Overall, this work demonstrates how NLRP1 monitors the cellular redox state, further illuminating an unexpected connection between the intracellular redox potential and the innate immune system.
中文翻译:

NLRP1 和氧化 TRX1 之间的相互作用涉及瞬时二硫键
NLRP1 是一种先天免疫受体,可检测病原体相关信号,组装成称为炎症小体的多蛋白结构,并触发称为细胞焦亡的促炎症形式的细胞死亡。我们之前发现硫氧还蛋白-1 的氧化形式(而非还原形式)直接与 NLRP1 结合并抑制炎症小体的形成。然而,NLRP1 仅与氧化形式的 TRX1 选择性结合的分子基础尚未确定。在这里,我们利用 AlphaFold-Multimer、定点诱变、硫醇捕获实验和质谱来揭示 NLRP1 上的特定半胱氨酸残基(人类中的 C427)与氧化的 TRX1 形成短暂的二硫键。总的来说,这项工作展示了 NLRP1 如何监测细胞氧化还原状态,进一步阐明细胞内氧化还原电位与先天免疫系统之间的意外联系。
更新日期:2024-01-11
中文翻译:

NLRP1 和氧化 TRX1 之间的相互作用涉及瞬时二硫键
NLRP1 是一种先天免疫受体,可检测病原体相关信号,组装成称为炎症小体的多蛋白结构,并触发称为细胞焦亡的促炎症形式的细胞死亡。我们之前发现硫氧还蛋白-1 的氧化形式(而非还原形式)直接与 NLRP1 结合并抑制炎症小体的形成。然而,NLRP1 仅与氧化形式的 TRX1 选择性结合的分子基础尚未确定。在这里,我们利用 AlphaFold-Multimer、定点诱变、硫醇捕获实验和质谱来揭示 NLRP1 上的特定半胱氨酸残基(人类中的 C427)与氧化的 TRX1 形成短暂的二硫键。总的来说,这项工作展示了 NLRP1 如何监测细胞氧化还原状态,进一步阐明细胞内氧化还原电位与先天免疫系统之间的意外联系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号