Green Synthesis and Catalysis Pub Date : 2024-01-12 , DOI: 10.1016/j.gresc.2024.01.005 Kejun Lin , Jianyong Lan , Lin Hao , Tingshun Zhu
|
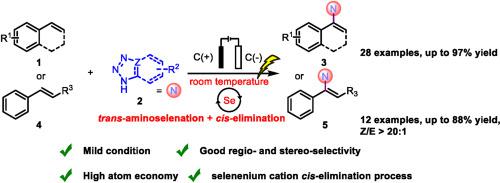
A selenium-catalyzed electrosynthesis involving regio- and stereo-selective N-olefination of azoles was developed. The room-temperature reaction was efficient (up to 97% yield) and compatible with various styrenes and azoles. Mechanistic study showed that the cascade reaction was triggered by the selenium-cation-mediated electrophilic trans-aminoselenation, and followed by an oxidative cis-elimination of selane. The electrosynthesis was also well compatible with the more challenging internal alkene substrates, giving the desired N-vinyl azoles in up to 88% yield and > 20:1 Z/E ratio.
中文翻译:

电化学 N-烯化用于乙烯基唑的区域和立体选择性合成
开发了一种涉及唑类区域选择性和立体选择性N-烯化的硒催化电合成方法。室温反应高效(产率高达 97%),并且与各种苯乙烯和唑类相容。机理研究表明,级联反应是由硒阳离子介导的亲电反氨基硒化引发的,随后是硒烷的氧化顺式消除。电合成还与更具挑战性的内部烯烃底物很好地兼容,以高达 88% 的产率和 > 20:1 Z/E比提供所需的N-乙烯基唑。



























 京公网安备 11010802027423号
京公网安备 11010802027423号