当前位置:
X-MOL 学术
›
Cell. Mol. Gastroenterol. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Noncanonical TRAIL Signaling Promotes Myeloid-Derived Suppressor Cell Abundance and Tumor Growth in Cholangiocarcinoma
Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2024-01-14 , DOI: 10.1016/j.jcmgh.2024.01.006 Emilien J. Loeuillard , Binbin Li , Hannah E. Stumpf , Jingchun Yang , Jessica R. Willhite , Jennifer L. Tomlinson , Fred Rakhshan Rohakhtar , Vernadette A. Simon , Rondell P. Graham , Rory L. Smoot , Haidong Dong , Sumera I. Ilyas
Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2024-01-14 , DOI: 10.1016/j.jcmgh.2024.01.006 Emilien J. Loeuillard , Binbin Li , Hannah E. Stumpf , Jingchun Yang , Jessica R. Willhite , Jennifer L. Tomlinson , Fred Rakhshan Rohakhtar , Vernadette A. Simon , Rondell P. Graham , Rory L. Smoot , Haidong Dong , Sumera I. Ilyas
|
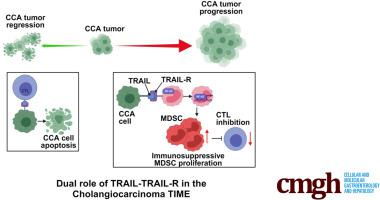
Proapoptotic tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) signaling as a cause of cancer cell death is a well-established mechanism. However, TRAIL-receptor (TRAIL-R) agonists have had very limited anticancer activity in human beings, challenging the concept of TRAIL as a potent anticancer agent. Herein, we aimed to define mechanisms by which TRAIL cancer cells can leverage noncanonical TRAIL signaling in myeloid-derived suppressor cells (MDSCs) promoting their abundance in murine cholangiocarcinoma (CCA). Multiple immunocompetent syngeneic, orthotopic models of CCA were used. Single-cell RNA sequencing and cellular indexing of transcriptomes and epitopes by sequencing of CD45 cells in murine tumors from the different CCA models was conducted. In multiple immunocompetent murine models of CCA, implantation of TRAIL murine cancer cells into mice resulted in a significant reduction in tumor volumes compared with wild-type mice. Tumor-bearing mice had a significant decrease in the abundance of MDSCs owing to attenuation of MDSC proliferation. Noncanonical TRAIL signaling with consequent nuclear factor-κB activation in MDSCs facilitated enhanced MDSC proliferation. Single-cell RNA sequencing and cellular indexing of transcriptomes and epitopes by sequencing of immune cells from murine tumors showed enrichment of a nuclear factor-κB activation signature in MDSCs. Moreover, MDSCs were resistant to TRAIL-mediated apoptosis owing to enhanced expression of cellular FLICE inhibitory protein, an inhibitor of proapoptotic TRAIL signaling. Accordingly, cellular FLICE inhibitory protein knockdown sensitized murine MDSCs to TRAIL-mediated apoptosis. Finally, cancer cell–restricted deletion of significantly reduced MDSC abundance and murine tumor burden. Our findings highlight the therapeutic potential of targeting TRAIL cancer cells for treatment of a poorly immunogenic cancer.
中文翻译:

非经典 TRAIL 信号传导促进胆管癌中骨髓源性抑制细胞的丰度和肿瘤生长
促凋亡肿瘤坏死因子相关的凋亡诱导配体 (TRAIL) 信号传导作为癌细胞死亡的原因是一个公认的机制。然而,TRAIL 受体(TRAIL-R)激动剂在人类中的抗癌活性非常有限,这对 TRAIL 作为有效抗癌剂的概念提出了挑战。在此,我们的目的是确定 TRAIL 癌细胞可以利用骨髓源性抑制细胞 (MDSC) 中的非经典 TRAIL 信号传导来促进其在小鼠胆管癌 (CCA) 中丰度的机制。使用了多个具有免疫活性的同基因、原位 CCA 模型。通过对来自不同 CCA 模型的小鼠肿瘤中的 CD45 细胞进行测序,进行了单细胞 RNA 测序以及转录组和表位的细胞索引。在多个具有免疫活性的 CCA 小鼠模型中,将 TRAIL 小鼠癌细胞植入小鼠体内,与野生型小鼠相比,肿瘤体积显着减小。由于 MDSC 增殖减弱,荷瘤小鼠的 MDSC 丰度显着下降。 MDSC 中非经典 TRAIL 信号传导以及随后的核因子-κB 激活促进了 MDSC 增殖的增强。单细胞 RNA 测序以及通过对小鼠肿瘤免疫细胞进行测序对转录组和表位进行细胞索引显示 MDSC 中核因子 κB 激活特征的富集。此外,由于细胞FLICE抑制蛋白(一种促凋亡TRAIL信号传导的抑制剂)的表达增强,MDSC对TRAIL介导的细胞凋亡具有抵抗力。因此,细胞 FLICE 抑制蛋白敲除使小鼠 MDSC 对 TRAIL 介导的细胞凋亡敏感。最后,癌细胞限制性删除显着降低了 MDSC 丰度和小鼠肿瘤负荷。我们的研究结果强调了靶向 TRAIL 癌细胞治疗免疫原性差的癌症的治疗潜力。
更新日期:2024-01-14
中文翻译:

非经典 TRAIL 信号传导促进胆管癌中骨髓源性抑制细胞的丰度和肿瘤生长
促凋亡肿瘤坏死因子相关的凋亡诱导配体 (TRAIL) 信号传导作为癌细胞死亡的原因是一个公认的机制。然而,TRAIL 受体(TRAIL-R)激动剂在人类中的抗癌活性非常有限,这对 TRAIL 作为有效抗癌剂的概念提出了挑战。在此,我们的目的是确定 TRAIL 癌细胞可以利用骨髓源性抑制细胞 (MDSC) 中的非经典 TRAIL 信号传导来促进其在小鼠胆管癌 (CCA) 中丰度的机制。使用了多个具有免疫活性的同基因、原位 CCA 模型。通过对来自不同 CCA 模型的小鼠肿瘤中的 CD45 细胞进行测序,进行了单细胞 RNA 测序以及转录组和表位的细胞索引。在多个具有免疫活性的 CCA 小鼠模型中,将 TRAIL 小鼠癌细胞植入小鼠体内,与野生型小鼠相比,肿瘤体积显着减小。由于 MDSC 增殖减弱,荷瘤小鼠的 MDSC 丰度显着下降。 MDSC 中非经典 TRAIL 信号传导以及随后的核因子-κB 激活促进了 MDSC 增殖的增强。单细胞 RNA 测序以及通过对小鼠肿瘤免疫细胞进行测序对转录组和表位进行细胞索引显示 MDSC 中核因子 κB 激活特征的富集。此外,由于细胞FLICE抑制蛋白(一种促凋亡TRAIL信号传导的抑制剂)的表达增强,MDSC对TRAIL介导的细胞凋亡具有抵抗力。因此,细胞 FLICE 抑制蛋白敲除使小鼠 MDSC 对 TRAIL 介导的细胞凋亡敏感。最后,癌细胞限制性删除显着降低了 MDSC 丰度和小鼠肿瘤负荷。我们的研究结果强调了靶向 TRAIL 癌细胞治疗免疫原性差的癌症的治疗潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号