当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical insights into photoinduced excited-state behaviors for the novel CHPPhl fluorophore: Effects of solvent polarity
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2024-01-14 , DOI: 10.1002/jccs.202300406 Chaozheng Li 1 , Mengmeng Hou 2 , Hao Dong 3 , Rivaille Liu 4
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2024-01-14 , DOI: 10.1002/jccs.202300406 Chaozheng Li 1 , Mengmeng Hou 2 , Hao Dong 3 , Rivaille Liu 4
Affiliation
|
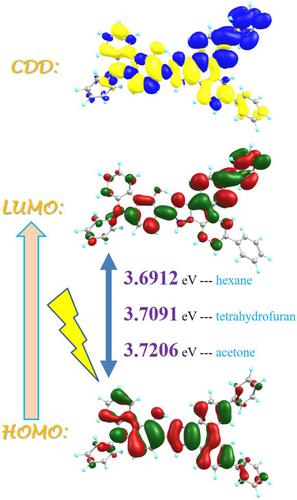
Inspired by the distinguished photochemical and photophysical properties of novel hydroxyl-substituted tetraphenylimidazole (HPI) derivatives that could be potentially applied across various disciplines, in this work, effects of solvent polarity on excited-state hydrogen bond effects and excited-state intramolecular proton transfer (ESIPT) reaction of 3-(6,9-Diphenyl-1H-phenanthro[9,10-d]imidazole-2-yl)-9-phenyl-9H-carbazol-4-ol (CHPPhl) are focused. By comparing the structural changes and infrared (IR) vibrational spectra of the E-HBT fluorophore in polar acetone, moderate polar tetrahydrofuran and non-polar hexane solvents, combined with the preliminary detection of hydrogen bond interaction by core–valence bifurcation (CVB) index, we can conclude that the hydrogen bond could be strengthened in S1 state, which is favorable for the occurrence of ESIPT reactions. The charge recombination behavior of hydrogen bond induced by photoexcitation also further illustrates this point. Via constructing potential energy curves (PECs) based on restrictive optimization and searching transition state (TS) form, we confirm change of surrounding solvent polarity has a regulatory effect on the ESIPT behavior for CHPPhl; that is, the lower the solvent polarity is more conducive to the ESIPT reaction.
中文翻译:

新型 CHPPhl 荧光团光诱导激发态行为的理论见解:溶剂极性的影响
受新型羟基取代的四苯基咪唑(HPI)衍生物的杰出光化学和光物理性质的启发,这些衍生物可能潜在地应用于各个学科,在这项工作中,溶剂极性对激发态氢键效应和激发态分子内质子转移的影响(重点关注 3-(6,9-二苯基-1H-菲[9,10-d]咪唑-2-基)-9-苯基-9H-咔唑-4-醇 (CHPPhl) 的 ESIPT) 反应。通过比较E-HBT荧光团在极性丙酮、中等极性四氢呋喃和非极性己烷溶剂中的结构变化和红外(IR)振动光谱,并结合核价分叉(CVB)指数初步检测氢键相互作用,我们可以得出结论,S 1态下氢键可以得到加强,这有利于ESIPT反应的发生。光激发引起的氢键电荷复合行为也进一步说明了这一点。通过基于限制性优化构建势能曲线(PEC)和搜索过渡态(TS)形式,我们证实周围溶剂极性的变化对CHPPhl的ESIPT行为具有调节作用;即溶剂极性越低越有利于ESIPT反应。
更新日期:2024-01-14
中文翻译:

新型 CHPPhl 荧光团光诱导激发态行为的理论见解:溶剂极性的影响
受新型羟基取代的四苯基咪唑(HPI)衍生物的杰出光化学和光物理性质的启发,这些衍生物可能潜在地应用于各个学科,在这项工作中,溶剂极性对激发态氢键效应和激发态分子内质子转移的影响(重点关注 3-(6,9-二苯基-1H-菲[9,10-d]咪唑-2-基)-9-苯基-9H-咔唑-4-醇 (CHPPhl) 的 ESIPT) 反应。通过比较E-HBT荧光团在极性丙酮、中等极性四氢呋喃和非极性己烷溶剂中的结构变化和红外(IR)振动光谱,并结合核价分叉(CVB)指数初步检测氢键相互作用,我们可以得出结论,S 1态下氢键可以得到加强,这有利于ESIPT反应的发生。光激发引起的氢键电荷复合行为也进一步说明了这一点。通过基于限制性优化构建势能曲线(PEC)和搜索过渡态(TS)形式,我们证实周围溶剂极性的变化对CHPPhl的ESIPT行为具有调节作用;即溶剂极性越低越有利于ESIPT反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号