当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic modelling of the cellular metabolic responses underpinning in vitro glycolysis assays
FEBS Open Bio ( IF 2.6 ) Pub Date : 2024-01-12 , DOI: 10.1002/2211-5463.13765 Nitin Patil 1, 2 , Zohreh Mirveis 1, 2 , Hugh J. Byrne 1
FEBS Open Bio ( IF 2.6 ) Pub Date : 2024-01-12 , DOI: 10.1002/2211-5463.13765 Nitin Patil 1, 2 , Zohreh Mirveis 1, 2 , Hugh J. Byrne 1
Affiliation
|
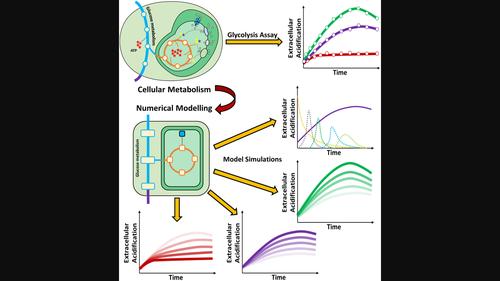
This study aims to demonstrate the benefits of augmenting commercially available, real-time, in vitro glycolysis assays with phenomenological rate equation-based kinetic models, describing the contributions of the underpinning metabolic pathways. To this end, a commercially available glycolysis assay, sensitive to changes in extracellular acidification (extracellular pH), was used to derive the glycolysis pathway kinetics. The pathway was numerically modelled using a series of ordinary differential rate equations, to simulate the obtained experimental results. The sensitivity of the model to the key equation parameters was also explored. The cellular glycolysis pathway kinetics were determined for three different cell-lines, under nonmodulated and modulated conditions. Over the timescale studied, the assay demonstrated a two-phase metabolic response, representing the differential kinetics of glycolysis pathway rate as a function of time, and this behaviour was faithfully reproduced by the model simulations. The model enabled quantitative comparison of the pathway kinetics of three cell lines, and also the modulating effect of two known drugs. Moreover, the modelling tool allows the subtle differences between different cell lines to be better elucidated and also allows augmentation of the assay sensitivity. A simplistic numerical model can faithfully reproduce the differential pathway kinetics for three different cell lines, with and without pathway-modulating drugs, and furthermore provides insights into the cellular metabolism by elucidating the underlying mechanisms leading to the pathway end-product. This study demonstrates that augmenting a relatively simple, real-time, in vitro assay with a model of the underpinning metabolic pathway provides considerable insights into the observed differences in cellular systems.
中文翻译:

支持体外糖酵解测定的细胞代谢反应的动力学模型
本研究旨在证明利用基于唯象速率方程的动力学模型增强市售实时体外糖酵解测定的好处,描述基础代谢途径的贡献。为此,使用对细胞外酸化(细胞外 pH)变化敏感的市售糖酵解测定法来推导糖酵解途径动力学。使用一系列常微分速率方程对该路径进行数值模拟,以模拟获得的实验结果。还探讨了模型对关键方程参数的敏感性。在非调节和调节条件下测定了三种不同细胞系的细胞糖酵解途径动力学。在研究的时间尺度上,该测定证明了两相代谢反应,代表了糖酵解途径速率随时间变化的微分动力学,并且模型模拟忠实地再现了这种行为。该模型能够定量比较三种细胞系的途径动力学,以及两种已知药物的调节作用。此外,建模工具可以更好地阐明不同细胞系之间的细微差异,并且还可以提高测定灵敏度。一个简单的数值模型可以忠实地重现三种不同细胞系的差异途径动力学,无论有或没有途径调节药物,并且通过阐明导致途径最终产物的潜在机制,进一步提供对细胞代谢的见解。这项研究表明,通过基础代谢途径模型增强相对简单的实时体外测定,可以深入了解细胞系统中观察到的差异。
更新日期:2024-01-12
中文翻译:

支持体外糖酵解测定的细胞代谢反应的动力学模型
本研究旨在证明利用基于唯象速率方程的动力学模型增强市售实时体外糖酵解测定的好处,描述基础代谢途径的贡献。为此,使用对细胞外酸化(细胞外 pH)变化敏感的市售糖酵解测定法来推导糖酵解途径动力学。使用一系列常微分速率方程对该路径进行数值模拟,以模拟获得的实验结果。还探讨了模型对关键方程参数的敏感性。在非调节和调节条件下测定了三种不同细胞系的细胞糖酵解途径动力学。在研究的时间尺度上,该测定证明了两相代谢反应,代表了糖酵解途径速率随时间变化的微分动力学,并且模型模拟忠实地再现了这种行为。该模型能够定量比较三种细胞系的途径动力学,以及两种已知药物的调节作用。此外,建模工具可以更好地阐明不同细胞系之间的细微差异,并且还可以提高测定灵敏度。一个简单的数值模型可以忠实地重现三种不同细胞系的差异途径动力学,无论有或没有途径调节药物,并且通过阐明导致途径最终产物的潜在机制,进一步提供对细胞代谢的见解。这项研究表明,通过基础代谢途径模型增强相对简单的实时体外测定,可以深入了解细胞系统中观察到的差异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号