当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Human Hsp70 Substrate-Binding Domains Recognize Distinct Client Proteins
Biochemistry ( IF 2.9 ) Pub Date : 2024-01-20 , DOI: 10.1021/acs.biochem.3c00531 Andrew J. Ambrose 1 , Christopher J. Zerio 1 , Jared Sivinski 1 , Xiaoyi Zhu 1 , Jack Godek 1 , Jonathan L. Sanchez 2 , May Khanna 3 , Rajesh Khanna 3 , Luke Lairson 4 , Donna D. Zhang 1 , Eli Chapman 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-01-20 , DOI: 10.1021/acs.biochem.3c00531 Andrew J. Ambrose 1 , Christopher J. Zerio 1 , Jared Sivinski 1 , Xiaoyi Zhu 1 , Jack Godek 1 , Jonathan L. Sanchez 2 , May Khanna 3 , Rajesh Khanna 3 , Luke Lairson 4 , Donna D. Zhang 1 , Eli Chapman 1
Affiliation
|
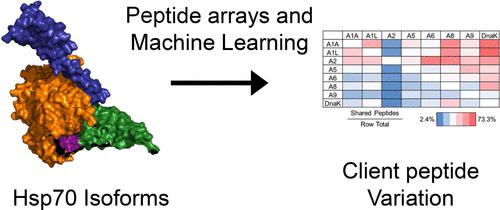
The 13 Hsp70 proteins in humans act on unique sets of substrates with diversity often being attributed to J-domain-containing protein (Hsp40 or JDP) cofactors. We were therefore surprised to find drastically different binding affinities for Hsp70-peptide substrates, leading us to probe substrate specificity among the 8 canonical Hsp70s from humans. We used peptide arrays to characterize Hsp70 binding and then mined these data using machine learning to develop an algorithm for isoform-specific prediction of Hsp70 binding sequences. The results of this algorithm revealed recognition patterns not predicted based on local sequence alignments. We then showed that none of the human isoforms can complement heat-shocked DnaK knockout Escherichia coli cells. However, chimeric Hsp70s consisting of the human nucleotide-binding domain and the substrate-binding domain of DnaK complement during heat shock, providing further evidence in vivo of the divergent function of the Hsp70 substrate-binding domains. We also demonstrated that the differences in heat shock complementation among the chimeras are not due to loss of DnaJ binding. Although we do not exclude JDPs as additional specificity factors, our data demonstrate substrate specificity among the Hsp70s, which has important implications for inhibitor development in cancer and neurodegeneration.
中文翻译:

人类 Hsp70 底物结合域识别不同的客户蛋白
人类中的 13 种 Hsp70 蛋白作用于一组独特的底物,其多样性通常归因于含有 J 结构域的蛋白(Hsp40 或 JDP)辅因子。因此,我们惊讶地发现 Hsp70 肽底物的结合亲和力截然不同,这使我们能够探测来自人类的 8 种典型 Hsp70 之间的底物特异性。我们使用肽阵列来表征 Hsp70 结合,然后使用机器学习挖掘这些数据,开发一种用于 Hsp70 结合序列的异构体特异性预测的算法。该算法的结果揭示了并非基于局部序列比对预测的识别模式。然后我们发现,没有一种人类亚型可以补充热休克 DnaK 敲除的大肠杆菌细胞。然而,在热休克过程中,由人核苷酸结合结构域和 DnaK 补体的底物结合结构域组成的嵌合 Hsp70,为体内 Hsp70 底物结合结构域的不同功能提供了进一步的证据。我们还证明了嵌合体之间热激互补的差异并不是由于 DnaJ 结合的丧失造成的。尽管我们不排除 JDP 作为额外的特异性因素,但我们的数据证明了 Hsp70 之间的底物特异性,这对癌症和神经退行性疾病的抑制剂开发具有重要意义。
更新日期:2024-01-20
中文翻译:

人类 Hsp70 底物结合域识别不同的客户蛋白
人类中的 13 种 Hsp70 蛋白作用于一组独特的底物,其多样性通常归因于含有 J 结构域的蛋白(Hsp40 或 JDP)辅因子。因此,我们惊讶地发现 Hsp70 肽底物的结合亲和力截然不同,这使我们能够探测来自人类的 8 种典型 Hsp70 之间的底物特异性。我们使用肽阵列来表征 Hsp70 结合,然后使用机器学习挖掘这些数据,开发一种用于 Hsp70 结合序列的异构体特异性预测的算法。该算法的结果揭示了并非基于局部序列比对预测的识别模式。然后我们发现,没有一种人类亚型可以补充热休克 DnaK 敲除的大肠杆菌细胞。然而,在热休克过程中,由人核苷酸结合结构域和 DnaK 补体的底物结合结构域组成的嵌合 Hsp70,为体内 Hsp70 底物结合结构域的不同功能提供了进一步的证据。我们还证明了嵌合体之间热激互补的差异并不是由于 DnaJ 结合的丧失造成的。尽管我们不排除 JDP 作为额外的特异性因素,但我们的数据证明了 Hsp70 之间的底物特异性,这对癌症和神经退行性疾病的抑制剂开发具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号