当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Picking Two out of Three: Defluorinative Annulation of Trifluoromethyl Alkenes for the Synthesis of Monofluorinated Carbo- and Heterocycles
The Chemical Record ( IF 6.6 ) Pub Date : 2024-01-22 , DOI: 10.1002/tcr.202300332 Jiahao Ling 1 , Lei Zhou 1
The Chemical Record ( IF 6.6 ) Pub Date : 2024-01-22 , DOI: 10.1002/tcr.202300332 Jiahao Ling 1 , Lei Zhou 1
Affiliation
|
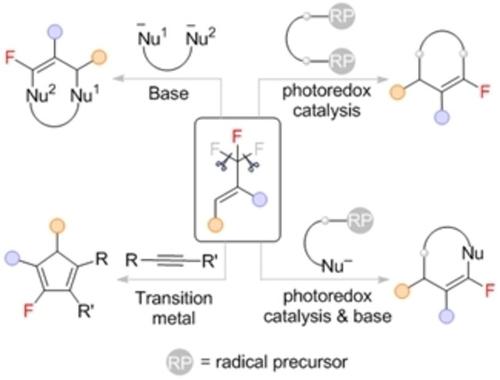
The increasing demand of organofluorine compounds in medicine, agriculture, and materials sciences makes sophisticated methods for their synthesis ever more necessary. Nowadays, not only the C−F bond formation but also the selective C−F bond cleavage of readily available poly- or perfluorine-containing compounds have become powerful tools for the effective synthesis of organofluorine compounds. The defluorinative cross-coupling of trifluoromethyl alkenes with various nucleophiles or radical precursors in an SN2’ manner is a convergent route to access gem-difluoroalkenes, which in turn react with nucleophiles or radical precursors via an SNV-type reaction. If the SNV reactions occur intramolecularly, the dual C−F bond cleavage of trifluoromethyl alkenes allows facile assembly of monofluorinated cyclic skeletons with structural complexity and diversity. In this personal account, we summarized the advances in this field on the basis of coupling and cyclization partners, including binucleophiles, alkynes, diradical precursors and radical precursors bearing a nucleophilic site. Accordingly, the annulation reactions can be achieved by base-mediated sequential SN2′/SNV reactions, transition metal catalyzed or mediated reactions, photoredox catalysis, and the combination of photocatalytic reactions with SNV reaction. In the context of seminal works of others in this field, a concise summary of the contributions of the authors is also offered.
中文翻译:

三选二:三氟甲基烯烃的脱氟环化用于合成单氟碳环和杂环
医学、农业和材料科学中对有机氟化合物的需求不断增长,使得复杂的合成方法变得更加必要。如今,不仅是CF键的形成,而且容易获得的含多氟或全氟化合物的选择性CF键断裂也已成为有效合成有机氟化合物的有力工具。三氟甲基烯烃与各种亲核试剂或自由基前体以 S N 2' 方式进行脱氟交叉偶联,是获得偕二氟烯烃的收敛途径,而偕二氟烯烃又通过 S N V 型反应与亲核试剂或自由基前体反应。如果 S N V 反应发生在分子内,三氟甲基烯烃的双 C−F 键断裂可以轻松组装具有结构复杂性和多样性的单氟化环状骨架。在这篇个人文章中,我们总结了该领域基于偶联和环化伙伴的进展,包括双亲核试剂、炔烃、双自由基前体和带有亲核位点的自由基前体。因此,环化反应可以通过碱介导的顺序S N 2'/S N V 反应、过渡金属催化或介导的反应、光氧化还原催化以及光催化反应与S N V 反应的组合来实现。在该领域其他人的开创性著作的背景下,还提供了作者贡献的简明摘要。
更新日期:2024-01-22
中文翻译:

三选二:三氟甲基烯烃的脱氟环化用于合成单氟碳环和杂环
医学、农业和材料科学中对有机氟化合物的需求不断增长,使得复杂的合成方法变得更加必要。如今,不仅是CF键的形成,而且容易获得的含多氟或全氟化合物的选择性CF键断裂也已成为有效合成有机氟化合物的有力工具。三氟甲基烯烃与各种亲核试剂或自由基前体以 S N 2' 方式进行脱氟交叉偶联,是获得偕二氟烯烃的收敛途径,而偕二氟烯烃又通过 S N V 型反应与亲核试剂或自由基前体反应。如果 S N V 反应发生在分子内,三氟甲基烯烃的双 C−F 键断裂可以轻松组装具有结构复杂性和多样性的单氟化环状骨架。在这篇个人文章中,我们总结了该领域基于偶联和环化伙伴的进展,包括双亲核试剂、炔烃、双自由基前体和带有亲核位点的自由基前体。因此,环化反应可以通过碱介导的顺序S N 2'/S N V 反应、过渡金属催化或介导的反应、光氧化还原催化以及光催化反应与S N V 反应的组合来实现。在该领域其他人的开创性著作的背景下,还提供了作者贡献的简明摘要。






























 京公网安备 11010802027423号
京公网安备 11010802027423号