Materials Science and Engineering: B ( IF 3.6 ) Pub Date : 2024-01-25 , DOI: 10.1016/j.mseb.2024.117201 Asmaa Khattari , Jaouad Bensalah , Amar Habsaoui , Zaki Safi , Nuha Wazzan , Avni Berisha , Abdelghani Hsini , Mustapha Tahaikt , Azzedine Elmidaoui
|
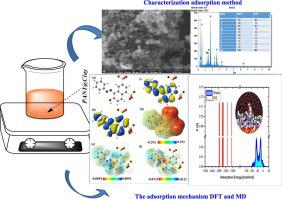
The purpose of this research was to determine if a natural clay treated with Polyaniline (PANI) might be used to effectively and affordably remove of anionic dyes from aqueous solutions, with a focus on Orange G dye (OG). After combining PANI with natural clay. We created a Polyaniline-clay composite after analyzing it with FTIR, XRD, SEM and EDS of the (PANI@Clay). Using batch adsorption tests, we compared the performance of PANI@Clay and raw clay at removing OG from aqueous solutions as a function of contact duration (10–360 min), initial concentration (50–500 mg/L), temperature (293–318 K), and pH (2–12). Results showed that PANI@Clay had a maximum equilibrium adsorption capacity of 67.35 mg/g, while raw-clay only managed 47.62 mg/g. Pseudo second-order and Langmuir models were both good fits for the kinetic behavior and the isotherms. According to the Gibbs energy and other thermodynamic parameters, OG adsorption onto PANI@Clay and raw clay was a spontaneous process that became more energy-intensive as temperature was raised. Overall, the results of this work show that PANI@Clay offers promising use as an adsorbent for cleaning water supplies of cationic dyes it explains the processes and thermodynamic features of OG adsorption, which were previously unknown onto PANI@Clay and raw clay. The structure of the OG dye was optimized at the B3LYP functional with 6-311+g(d,p) set in aqueous solution. The global and local chemical reactivity descriptors were calculated to suggest a suitable mechanism for removal process.
中文翻译:

使用新型低成本吸附剂 PANI@Clay 复合材料有效去除废水中的 OG 染料:等温动力学和热力学模型的见解,使用密度泛函理论 DFT/MC/MD 进行研究
本研究的目的是确定用聚苯胺 (PANI) 处理的天然粘土是否可用于有效且经济地去除水溶液中的阴离子染料,重点是橙色 G 染料 (OG)。将PANI与天然粘土结合后。经过对 ( PANI@Clay )的 FTIR、XRD、SEM 和 EDS 分析后,我们创建了聚苯胺-粘土复合材料。通过批量吸附测试,我们比较了PANI@Clay和生粘土从水溶液中去除 OG的性能,作为接触时间(10-360 分钟)、初始浓度(50-500 mg/L)、温度(293- 318 K) 和 pH (2–12)。结果表明,PANI@Clay的最大平衡吸附容量为 67.35 mg/g,而生粘土仅为 47.62 mg/g。伪二阶模型和 Langmuir 模型都非常适合动力学行为和等温线。根据吉布斯能和其他热力学参数,OG在PANI@Clay和生粘土上的吸附是一个自发过程,随着温度的升高,该过程变得更加能量密集。总体而言,这项工作的结果表明,PANI@Clay作为清洁阳离子染料供水的吸附剂具有广阔的应用前景,它解释了 OG 吸附的过程和热力学特征,而这在PANI@Clay和生粘土上以前是未知的。OG染料的结构在B 3 LYP官能团上进行了优化,在水溶液中设置了6-311+g(d,p)。计算了全局和局部化学反应性描述符,以提出合适的去除过程机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号