当前位置:
X-MOL 学术
›
Adv. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Overcoming colloidal nanoparticle aggregation in biological milieu for cancer therapeutic delivery: Perspectives of materials and particle design
Advances in Colloid and Interface Science ( IF 15.6 ) Pub Date : 2024-01-26 , DOI: 10.1016/j.cis.2024.103094 Shi Huan Lim , Tin Wui Wong , Tay Wei Xian
Advances in Colloid and Interface Science ( IF 15.6 ) Pub Date : 2024-01-26 , DOI: 10.1016/j.cis.2024.103094 Shi Huan Lim , Tin Wui Wong , Tay Wei Xian
|
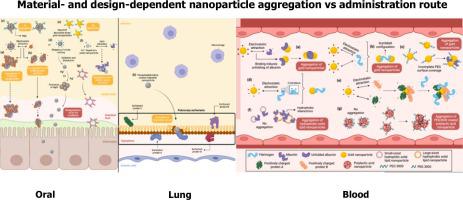
Nanoparticles as cancer therapeutic carrier fail in clinical translation due to complex biological environments in vivo consisting of electrolytes and proteins which render nanoparticle aggregation and unable to reach action site. This review identifies the desirable characteristics of nanoparticles and their constituent materials that prevent aggregation from site of administration (oral, lung, injection) to target site. Oral nanoparticles should ideally be 75–100 nm whereas the size of pulmonary nanoparticles minimally affects their aggregation. Nanoparticles generally should carry excess negative surface charges particularly in fasting state and exert steric hindrance through surface decoration with citrate, anionic surfactants and large polymeric chains (polyethylene glycol and polyvinylpyrrolidone) to prevent aggregation. Anionic as well as cationic nanoparticles are both predisposed to protein corona formation as a function of biological protein isoelectric points. Their nanoparticulate surface composition as such should confer hydrophilicity or steric hindrance to evade protein corona formation or its formation should translate into steric hindrance or surface negative charges to prevent further aggregation. Unexpectedly, smaller and cationic nanoparticles are less prone to aggregation at cancer cell interface favoring endocytosis whereas aggregation is essential to enable nanoparticles retention and subsequent cancer cell uptake in tumor microenvironment. Present studies are largely conducted in vitro with simplified simulated biological media. Future aggregation assessment of nanoparticles in biological fluids that mimic that of patients is imperative to address conflicting materials and designs required as a function of body sites in order to realize the future clinical benefits.
中文翻译:

克服生物环境中的胶体纳米颗粒聚集以实现癌症治疗:材料和颗粒设计的视角
由于体内由电解质和蛋白质组成的复杂生物环境导致纳米颗粒聚集并且无法到达作用位点,因此纳米颗粒作为癌症治疗载体在临床转化中失败。本综述确定了纳米颗粒及其组成材料的理想特性,可防止从给药部位(口服、肺部、注射)聚集到目标部位。理想情况下,口服纳米颗粒应为 75-100 nm,而肺部纳米颗粒的大小对其聚集的影响最小。纳米颗粒通常应携带过量的表面负电荷,特别是在禁食状态下,并通过柠檬酸盐、阴离子表面活性剂和大聚合物链(聚乙二醇和聚乙烯吡咯烷酮)进行表面装饰来施加空间位阻,以防止聚集。作为生物蛋白质等电点的函数,阴离子和阳离子纳米粒子都易于形成蛋白质电晕。它们的纳米颗粒表面组成本身应赋予亲水性或空间位阻以避免蛋白质冠的形成,或其形成应转化为空间位阻或表面负电荷以防止进一步聚集。出乎意料的是,较小的阳离子纳米颗粒不太容易在癌细胞界面处聚集,有利于内吞作用,而聚集对于使纳米颗粒在肿瘤微环境中保留和随后的癌细胞摄取至关重要。目前的研究主要是在体外使用简化的模拟生物介质进行的。未来对生物体液中纳米颗粒的聚集进行模拟患者的聚集评估,必须解决作为身体部位功能所需的相互冲突的材料和设计,以实现未来的临床益处。
更新日期:2024-01-26
中文翻译:

克服生物环境中的胶体纳米颗粒聚集以实现癌症治疗:材料和颗粒设计的视角
由于体内由电解质和蛋白质组成的复杂生物环境导致纳米颗粒聚集并且无法到达作用位点,因此纳米颗粒作为癌症治疗载体在临床转化中失败。本综述确定了纳米颗粒及其组成材料的理想特性,可防止从给药部位(口服、肺部、注射)聚集到目标部位。理想情况下,口服纳米颗粒应为 75-100 nm,而肺部纳米颗粒的大小对其聚集的影响最小。纳米颗粒通常应携带过量的表面负电荷,特别是在禁食状态下,并通过柠檬酸盐、阴离子表面活性剂和大聚合物链(聚乙二醇和聚乙烯吡咯烷酮)进行表面装饰来施加空间位阻,以防止聚集。作为生物蛋白质等电点的函数,阴离子和阳离子纳米粒子都易于形成蛋白质电晕。它们的纳米颗粒表面组成本身应赋予亲水性或空间位阻以避免蛋白质冠的形成,或其形成应转化为空间位阻或表面负电荷以防止进一步聚集。出乎意料的是,较小的阳离子纳米颗粒不太容易在癌细胞界面处聚集,有利于内吞作用,而聚集对于使纳米颗粒在肿瘤微环境中保留和随后的癌细胞摄取至关重要。目前的研究主要是在体外使用简化的模拟生物介质进行的。未来对生物体液中纳米颗粒的聚集进行模拟患者的聚集评估,必须解决作为身体部位功能所需的相互冲突的材料和设计,以实现未来的临床益处。



























 京公网安备 11010802027423号
京公网安备 11010802027423号