Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2024-01-26 , DOI: 10.1016/j.bmcl.2024.129620 Gabriela Mazur , Katarzyna Pańczyk-Straszak , Karolina Krysińska , Karolina Niemiec , Anna Waszkielewicz
|
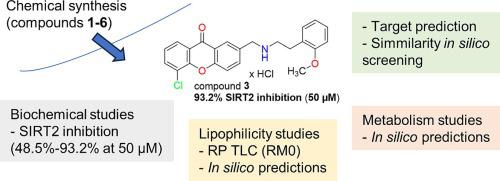
Six amino derivatives of xanthone were obtained via chemical synthesis. Biochemical studies revealed their SIRT2 inhibitory activity ranging from 48.5 % (compound 4, 5-chloro-2-((4-(3-methoxyphenyl)piperazin-1-yl)methyl)-9H-xanthen-9-one hydrochloride) to 93.2 % (compound 3, 5-chloro-2-(((2-methoxyphenethyl)amino)methyl)-9H-xanthen-9-one hydrochloride). The structure–activity analysis showed favourable properties of secondary amines relative to tertiary piperazine derivatives. The tested compounds do not possess additional SIRT1 activating activity and no antioxidant activity (DPPH in vitro assay). Comprehensive analysis of the lipophilicity of the obtained compounds was also performed. For compound 3 potential molecular targets and similar active compounds were predicted in order to facilitate further research in this group of compounds.
中文翻译:

作为有效的 Sirtuin 2 抑制剂的新型呫吨酮衍生物
通过化学合成得到了六种氧杂蒽酮的氨基衍生物。生化研究表明,它们的 SIRT2 抑制活性范围为 48.5 %(化合物4 , 5-氯-2-((4-(3-甲氧基苯基)哌嗪-1-基)甲基)-9H-xanthen-9-one 盐酸盐)到 93.2 %(化合物3,5-氯-2-(((2-甲氧基苯乙基)氨基)甲基)-9H-呫吨-9-酮盐酸盐)。结构-活性分析显示仲胺相对于叔哌嗪衍生物具有有利的性质。测试的化合物不具有额外的 SIRT1 激活活性,并且没有抗氧化活性(DPPH体外测定)。还对所得化合物的亲脂性进行了综合分析。对于化合物3,预测了潜在的分子靶点和类似的活性化合物,以促进对该组化合物的进一步研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号