当前位置:
X-MOL 学术
›
Adv. Therap.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A 3D In Vitro Triculture Hybrid Model Recapitulating Tumor Stromal Interaction of Triple-Negative Breast Cancer as a High Throughput Anticancer Drug Screening Platform
Advanced Therapeutics ( IF 4.6 ) Pub Date : 2024-01-26 , DOI: 10.1002/adtp.202300450 Chitra Jaiswal 1 , Biman B. Mandal 1, 2, 3
Advanced Therapeutics ( IF 4.6 ) Pub Date : 2024-01-26 , DOI: 10.1002/adtp.202300450 Chitra Jaiswal 1 , Biman B. Mandal 1, 2, 3
Affiliation
|
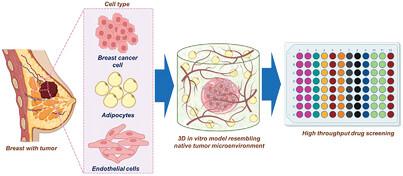
Breast cancer (BC) progression is substantially driven by cellular cross-talk between tumor and stromal cells within the tumor microenvironment (TME). However, lack of precise recapitulation of the heterocellular complexity, interactions in oversimplified 2D models, and physiological differences in animal models lead to discrepancy in anticancer drug response. Three-dimensional (3D) in vitro bioengineered models that physiologically resemble in vivo TME are apt to overcome the discrepancy in the preclinical drug testing outcome. Here, a compartmentalized 3D in vitro triculture triple-negative breast cancer (TC-TNBC) model is bioengineered using silk-fibroin (SF) scaffold and GelMA hydrogel for recapitulating stromal and tumor extracellular niche, respectively. The model features the cellular heterogeneity of stromal niche by incorporating most representative cell types in breast tissue, i.e., adipocytes and endothelial cells, and MDA-MB-231 cells in the tumor niche. The TC-TNBC model exhibits enhanced tumorigenic potential in the presence of stromal cells. Screening of model anticancer drugs (doxorubicin (Dox) and cisplatin (Cis)) exhibits an increase in IC50 concentration in TC-TNBC as compared to monoculture, suggesting crucial role of stromal cells in drug response. Drug sensitivity and cytotoxicity behavior mimick the in vivo like drug response. This model provides a facile and adaptable platform to represent tumor-stromal heterogeneity and high-throughput anticancer drug screening.
中文翻译:

3D 体外三培养混合模型概括三阴性乳腺癌的肿瘤基质相互作用作为高通量抗癌药物筛选平台
乳腺癌(BC)的进展主要是由肿瘤微环境(TME)内肿瘤和基质细胞之间的细胞串扰驱动的。然而,缺乏对异细胞复杂性的精确概括、过于简化的二维模型中的相互作用以及动物模型中的生理差异导致抗癌药物反应的差异。三维 (3D) 体外生物工程模型在生理上类似于体内 TME,易于克服临床前药物测试结果的差异。在这里,使用丝素蛋白 (SF) 支架和 GelMA 水凝胶分别对基质和肿瘤细胞外生态位进行生物工程,构建了分隔的 3D 体外三培养三阴性乳腺癌 (TC-TNBC) 模型。该模型通过纳入乳腺组织中最具代表性的细胞类型(即脂肪细胞和内皮细胞)以及肿瘤生态位中的 MDA-MB-231 细胞来表征基质生态位的细胞异质性。 TC-TNBC 模型在基质细胞存在的情况下表现出增强的致瘤潜力。模型抗癌药物(阿霉素 (Dox) 和顺铂 (Cis))的筛选显示,与单一培养物相比,TC-TNBC 中的IC 50浓度有所增加,表明基质细胞在药物反应中的关键作用。药物敏感性和细胞毒性行为模仿体内药物反应。该模型提供了一个简单且适应性强的平台来表示肿瘤基质异质性和高通量抗癌药物筛选。
更新日期:2024-01-26
中文翻译:

3D 体外三培养混合模型概括三阴性乳腺癌的肿瘤基质相互作用作为高通量抗癌药物筛选平台
乳腺癌(BC)的进展主要是由肿瘤微环境(TME)内肿瘤和基质细胞之间的细胞串扰驱动的。然而,缺乏对异细胞复杂性的精确概括、过于简化的二维模型中的相互作用以及动物模型中的生理差异导致抗癌药物反应的差异。三维 (3D) 体外生物工程模型在生理上类似于体内 TME,易于克服临床前药物测试结果的差异。在这里,使用丝素蛋白 (SF) 支架和 GelMA 水凝胶分别对基质和肿瘤细胞外生态位进行生物工程,构建了分隔的 3D 体外三培养三阴性乳腺癌 (TC-TNBC) 模型。该模型通过纳入乳腺组织中最具代表性的细胞类型(即脂肪细胞和内皮细胞)以及肿瘤生态位中的 MDA-MB-231 细胞来表征基质生态位的细胞异质性。 TC-TNBC 模型在基质细胞存在的情况下表现出增强的致瘤潜力。模型抗癌药物(阿霉素 (Dox) 和顺铂 (Cis))的筛选显示,与单一培养物相比,TC-TNBC 中的IC 50浓度有所增加,表明基质细胞在药物反应中的关键作用。药物敏感性和细胞毒性行为模仿体内药物反应。该模型提供了一个简单且适应性强的平台来表示肿瘤基质异质性和高通量抗癌药物筛选。



























 京公网安备 11010802027423号
京公网安备 11010802027423号